| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Review
Volume 18, Number 3, June 2025, pages 93-100
Eflornithine for the Chemoprevention of Luminal Gastrointestinal Neoplasms: A Systematic Review
Ambar Godoya, Daniela Montalvan-Sanchezb, Fortunato S. Principe-Menesesc, Adrian Riva-Moscosoc, d, Leandro Sierrae, Gloria Erazof, Carlos Avilag, Mirian Ramirez-Rojash, Roberto Gironi, Daniel A. Guifarroj, k
aIndiana University School of Medicine, Department of Medicine, Indianapolis, IN, USA
bDepartamento de Medicina Interna, Universidad Nacional Autonoma de Honduras, Tegucigalpa, Honduras
cEscuela de Medicina, Universidad Peruana de Ciencias Aplicadas, Lima, Peru
dHospital II-1 Alto Mayo EsSalud, San Martin, Peru
eDepartment of Medicine, Cleveland Clinic Foundation, Cleveland, OH, USA
fTexas Tech University Health Sciences Center, Lubbock, TX, USA
gManatee Memorial Hospital, Bradenton, FL, USA
hRuth Lilly Medical Library, Indiana University School of Medicine, Indianapolis, IN, USA
iDepartment of Medicine, University of Nevada Reno, Reno, NV, USA
jDepartment of Internal Medicine, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, USA
kCorresponding Author: Daniel A. Guifarro, Department of Internal Medicine, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, USA
Manuscript submitted January 8, 2025, accepted April 12, 2025, published online June 4, 2025
Short title: DFMO Chemoprevention in Luminal GI Neoplasia
doi: https://doi.org/10.14740/gr1801
| Abstract | ▴Top |
Background: Gastrointestinal (GI) tract malignancies represent a significant global health burden, being major contributors to cancer-related morbidity and mortality globally, with over 7.7 million cases reported. While aspirin is a well-studied chemopreventive agent for GI neoplasms, its use may be limited due to the underlying bleeding risk. Eflornithine (DFMO) is an inhibitor of the ornithine decarboxylase (ODC) which inhibits polyamine synthesis, and has shown promise as an alternative chemopreventive agent, particularly in animal studies and limited clinical trials.
Methods: Following PRISMA guidelines, we conducted a systematic review of studies evaluating DFMO alone or in combination for chemoprevention in premalignant GI lesions including chronic gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia. The protocol was registered in Prospero (CRD42022309307). Randomized controlled trials (RCTs) and cohort studies in English or Spanish were included.
Results: Nine studies (six RCTs and three phase I-II trials) met inclusion criteria. Phase I-II trials involving Barrett’s esophagus and gastric cancer did not report significant benefits. Phase III-IV trials combining DFMO with nonsteroidal anti-inflammatory drugs (NSAIDs) were associated with reductions in adenoma recurrence, size, and polyamine levels in high-risk GI cancer populations. Side effects included ototoxicity, reversible upon discontinuation, and mild GI events, both occurring at higher doses.
Conclusion: While aspirin remains a frontline chemopreventive agent for GI neoplasms, this review shows that phase III-IV trials suggest promising outcomes in combination with NSAIDs, warranting further investigation. Notably, DFMO’s low cost and favorable toxicity profile may position it as a viable alternative, emphasizing the need for additional RCTs to delineate its efficacy and safety in GI cancer prevention. Further investigation into DFMO’s optimal dosage, duration, and side effect management is essential to establish it as a safe and effective chemopreventive agent.
Keywords: Chemoprophylaxis; Gastrointestinal luminal cancer; Eflornithine; DFMO
| Introduction | ▴Top |
Gastrointestinal (GI) tract malignancies are an important cause of cancer-related morbidity and mortality worldwide. Colorectal cancers (CRCs) are reported as the second major cause of deaths related to cancer, while stomach cancer is the fourth major cause. In 2020, colorectal, esophageal, and stomach cancers had a combined prevalence of 7.7 million, causing 2.2 million deaths [1, 2]. Thus, a significant amount of research has been invested in the prevention of these malignancies.
Currently, aspirin is the best-studied medication for the prevention of GI neoplasms. In 2016, the United States Preventative Service Task Force (USPSTF) recommended low-dose aspirin for primary prevention of colon and rectal cancers (grade B recommendation), for 50- to 59-year-old patients without increased bleeding risk for at least 10 years. A grade C recommendation was made for those aged 60 - 69 [3]. This recommendation followed research suggesting that CRC risk is associated with chronic inflammation. Specifically, cyclooxgenase-2 (COX-2) activation has been theorized to play a key role in CRC progression through its role in inhibiting apoptosis and promoting angiogenesis, tumor proliferation, and invasion. Due to its inhibitory effects on COX-2, aspirin was thought to be a candidate for chemoprevention of GI malignancies in specific populations [4-6]. Chemoprevention is the use of drugs to prevent or delay the development of malignancy. Ng et al studied 799 patients with stage 3 colon cancer who were undergoing adjuvant chemotherapy, and reported that aspirin was associated with slight improvement in recurrence-free survival with a hazard ratio (HR) of 0.51 (95% confidence interval (CI): 0.28 - 0.95) [7]. For those solely using aspirin for CRC prevention, the benefits were more often seen with long-term use. A 2010 study following participants of five randomized controlled trials (RCTs) treated with aspirin 75 - 300 mg daily found a reduction in CRC mortality at 20 years of treatment (HR: 0.65, 95% CI: 0.48 - 0.88) [8]. Other NSAIDs, particularly naproxen, sulindac, and celecoxib have also been studied; however, the exact dose needed to treat a population and long-term follow-up studies are still needed [9]. Since the USPSTF recommendation, follow-up studies and additional reporting from existing studies have emerged. In particular, the ASPREE trial, an RCT following 19,114 participants aged 65 - 70 for a median of 4.7 years, found no significant difference for all cancer rates in the aspirin administration and placebo group (HR: 1.04, 95% CI: 0.95 - 1.14). Nonetheless, the patient group that received treatment with aspirin was found to have higher rates of metastatic cancer at the time of diagnosis than the placebo group [10]. The positive impact of aspirin treatment on the risk of cancer may be found with long-term treatment. However, considering the findings of the ASPREE trial along with the known bleeding risk associated with aspirin use, it seems that starting aspirin for chemoprevention could be a highly individualized decision.
This paper is investigating the utility of eflornithine or difluoromethylornithine (DFMO) as a possible medication for the chemoprevention of GI luminal malignancies. DFMO is currently marketed for its uses in facial hair reduction and West-African trypanosomiasis. The mechanism of action is irreversible inhibition of ornithine decarboxylase (ODC), which is a rate-limiting factor involved in the synthesis of polyamines. All mammalian cells depend on ODC to produce polyamine necessary for the synthesis of DNA, RNA, and proteins. It is one of the enzymes transcriptionally activated by the MYC oncogene to convert ornithine to putrescine, which then provides the propylamine group to spermidine synthetase (SRM). The polyamine group is subsequently transferred from SRM to the eukaryotic translocation initiation factor 5A2 (9eIF5A2), which has been shown to have oncogenic potential [11]. In gastric mucosa, DFMO treatment in gerbils that had been infected with H. pylori was associated with decreased gastric epithelial dysplasia and gastric carcinogenesis. These effects were thought to be attributed to the inhibition of polyamine synthesis and oxidation [12]. In a study of 10 participants with Barrett’s esophagus, mucosal biopsies of patients treated with DFMO for 3, 6, and 12 months showed a reduction in mucosal polyamines [13]. Moreover, DFMO treatment was associated with a down-regulation of transcription factors associated with cell proliferation. In rodent models, DFMO given at small doses was shown to inhibit intestinal and colon carcinogenesis [14-17].
A systematic review was done with the goal of studying the efficacy and safety of DFMO as a single agent or in combination for luminal GI neoplasm prevention.
| Materials and Methods | ▴Top |
This protocol is developed following the guideline of Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocols [16]. The protocol for this systematic review was registered in Prospero with CRD42022309307.
Inclusion criteria
We included any study design evaluating DFMO alone or in combination use as chemoprevention for premalignant lesions, and RCTs and cohort studies published in English or Spanish. We excluded observational studies and non-RCTs.
Types of participants
Participants were all adults (male and female), over 18 years old, with a premalignant GI lesion that was treated with DFMO as a chemopreventive agent. The primary outcome was the progression of premalignant lesions. The secondary outcome was to assess the toxicity and tolerability of DFMO.
Search strategy
A comprehensive search strategy was developed and run in six databases: Medline (Ovid), Embase (Elsevier), Cochrane Library, Web of Science Core Collection, Scopus, and the US National Institutes of Health (NIH) Clinical Trials Registry platform. The search consisted of a combination of keywords and subject headings used in the title and the abstract as keywords. Search terms focused on GI tumor, cancer or neoplasm, and DFMO-related terms and synonyms. Limits were added to the searches to retrieve only humans and English-language studies. The search was executed in each database from inception to March 28, 2023. The final Medline strategy is provided (Supplementary Material 1, gr.elmerpub.com). The results from all databases used were aggregated in Endnote and de-duplicated using the Covidence tool [18] for further screening. All searches in this study were developed and executed by a medical librarian (MR).
| Results | ▴Top |
Baseline characteristics
We identified 1,790 publications. After removing duplicates and screening phases, we selected articles for full-text screening. Finally, nine studies were included in the meta-analysis (Fig. 1). Six studies were RCTs and three were phase I-II trials.
 Click for large image | Figure 1. Eflornithine study selection flow diagram (PRISMA 2009). |
Outcomes
Phase I-II trials
A review of randomized studies on Barrett’s esophagus or low-grade dysplasia involving 10 subjects showed no significant benefits during endoscopic monitoring, nor any efficacy in reducing the tissue content of polyamines, and one patient experienced subclinical ototoxicity [13]. A phase I-II study of chemotherapy plus DFMO compared to chemotherapy alone with seven gastric cancer subjects showed no benefits, and the major side effect was reversible ototoxicity after stopping treatment with DFMO [19]. High-dose intravenous DFMO in a phase II trial for CRC was not associated with any response in 14 subjects that received a monthly schedule of 3 weeks of treatment and 1 week without treatment [20].
Phase III-IV trials
The publications ranged from 2008 to 2020. Patients were followed up from 6 to 36 months, and the intervention arm was DFMO and sulindac or aspirin or celecoxib. The primary outcome was disease progression, adenoma number, or adenoma size. There were four RCTs using DFMO combined with NSAIDs for colon and rectal cancer chemoprevention demonstrating a reduction in reported adenomas (Table 1) [21-26]. Additionally, ototoxicity was reported as the most common side effect of therapy in five studies, which was more common at high doses and was reversible upon discontinuation. Anemia was reported as an adverse effect in three studies, and minor GI side effects were reported in all studies. There were several undergoing studies evaluating chemoprevention with DFMO for CRC and gastric cancer.
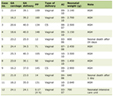 Click to view | Table 1. General Characteristics of the Included Studies |
The quality of the studies was measured with the modified Newcastle-Ottawa scale (mNOS), ranging from 5 to 9 points. Loss of points in most studies corresponded to low health research inclusivity (HRI) population representativeness, non-reported characteristics of salient patients, and limited follow-up.
| Discussion | ▴Top |
This systematic review thoroughly analyzes recent studies involving adults with either familial adenomatous polyposis (FAP) or prior history of colorectal adenomas to highlight the possible chemoprophylactic role of either DFMO as a single agent or its combination with NSAIDs for the prevention of luminal GI neoplasms.
Two of the studies considered FAP, as the presence of multiple adenomas and accumulation of mutations occurring in the GI tract serve as a good model syndrome for CRC chemoprevention [16]. These studies included subjects with FAP and pathogenic variants of APC independently of colectomy status (including pre-colectomy and post-colectomy with preserved colon segments subgroups) [21, 27].
Yang et al in their study reported the use of DFMO as a chemoprevention therapy for CRC. They found that DFMO combination significantly reduces the incidence of adenomas in patients with recurrent CRC. However, there was no difference in control of disease progression in FAP with DFMO combination therapy [28].
Multiple prior research suggests that many cancers produce COX-2 to induce angiogenesis and inhibit apoptosis in early stages [29-31]. While inhibition of COX-2 aids in decreasing the inflammatory process, such as in esophageal cancer and Barret’s esophagus patients [32], polyamines have also been shown to be essential for cancer cells survival [33]. Recently, a study done on Barrett’s esophagus showed that polyamine expression was higher in intestinal metaplasia compared to normal gastric and squamous mucosa [34]. This finding suggests a correlation between elevated polyamine levels and tumorigenesis and cancer cell proliferation. Conversely, the inhibition of polyamine synthesis has been associated with a decrease in cancerous cell growth [35-37].
Prior and ongoing research is being conducted to study the role of chemoprophylaxis in preventing and controlling luminal GI neoplasms. Some chemoprophylactic benefits include decreasing GI cancer incidence, mortality, and latency period. These benefits subsequently lead to a decrease in GI cancer burden and cost to the patient plus healthcare system. NSAIDs and aspirin are widely investigated as possible agents for chemoprevention, especially in GI cancers, but until recently, the use of DFMO by itself or concomitantly with NSAIDs has not been thoroughly researched.
Aspirin ingestion is associated with reduced stomach, colorectal, and esophageal carcinomas as reported in multiple studies [38-40]. For example, a study showed that aspirin use is associated with lower risk of colon cancer, taking into account the dose and duration of exposure [41, 42]. Thus, it has been thought that aspirin could be playing a role in both cancer prevention and progression via its COX-2 inhibitory role [43]. Furthermore, another study supporting this theory of inhibiting chemically induced carcinogenesis highlighted a decrease in incidence and multiplicity of colonic tumors with NSAIDs use, regardless of NSAIDs agent type or treatment timing [44].
Multiple studies have been performed on the anti-tumor effect that DFMO has on pancreatic, skin, breast, prostate, blood, and ovarian cancers, due to its action on apoptotic signaling [45, 46]. However, few studies are being conducted on its chemoprophylactic effect, either as a single agent or its combination with other agents, mostly NSAIDs. DFMO works by irreversibly inhibiting polyamine metabolism, specifically the overexpressed enzyme ODC, which is the rate-limiting enzyme for polyamines synthesis that is present in patients with FAP [47, 48]. Taking this into account, DFMO could have a role in polyp prevention in this population [8, 9].
Different trials have been carried out on the minimal effective dosing and potential toxicities of DFMO. In the analyzed studies, the minimal dose of DFMO ranged from 500 to 750 mg daily [17, 21-24, 27]. The average minimal dosing needed to achieve potential effective chemoprevention in any particular organ has been estimated at 0.5 g/m2/day, which causes the reduction in polyamine levels [17, 23]. Interestingly, in some studies, it was found that DFMO causes a decrease in colorectal mucosal ratios of polyamines with doses as low as 0.1 g/m2 daily for 4 weeks [17, 49].
All of the studies in Table 1 evaluated the efficacy of DFMO for the prevention of colonic polyposis and neoplasms in an effort to reduce CRC incidence and improve outcomes.
In Burke et al’s study, it was found that the use of DFMO resulted in 40% of patients having FAP progression compared to progression of disease in 32% and 38% DFMO-sulindac and sulindac groups, respectively [21]. Another interesting finding was that the average time to progression was the longest in the DFMO-sulindac combined group of 32.3 months, compared to sulindac-only and DFMO-only groups, which had progression rates of 23.6 and 21.8 months, respectively [21]. There was an increase in time to progression, but not to a level of statistical significance. DMFO has also been shown to delay or prevent the need for lower gastrointestinal tract surgery in patients with FAP as described by Balaguer et al [24].
In the clinical trial by Sinicrope et al, the placebo arm showed similar rate of recurrence to the study arm including DFMO and sulindac (41.1% rate after treatment for 2 - 39 months) [25]. DFMO and aspirin combination had an association with significant reduction of rectal aberrant crypt foci count in comparison to patients that were in the placebo arm (P = 0.036) [25].
The trial by Lynch et al revealed that the celecoxib and placebo group showed a 1% mean reduction in comparison to the DFMO and celecoxib group reporting a 13% reduction of polyps measuring a minimum of 2 mm [22].
In the trial by Meyskens et al, the research examined the effects of combining low doses of DFMO and sulindac to reduce the recurrence of colorectal adenomas identified through standard colonoscopy. Among the placebo group, 53 patients (41.1%) developed at least one adenoma, compared to only 17 patients (12.3%) in the treatment group (P < 0.001). Additionally, 11 patients in the placebo group had advanced adenomas, whereas only one case of advanced adenoma was observed in the DFMO plus sulindac group (P < 0.001) [23].
In the trial by Morgan et al, upon evaluation of DNA damage, patients receiving eflornithine demonstrated a slight increase in %pH2AX at 6 and 18 months; however, a significant reduction in this marker was noted by the end of the study period (EoS), particularly in the analysis of adjacent time points (P = 0.012). These findings support the safety and tolerability of eflornithine in individuals with gastric premalignant conditions in Latin America and suggest a potential role in mitigating long-term DNA damage following treatment completion [26].
Another study found that combining low doses of piroxicam and DFMO was more effective in reducing the incidence and multiplicity of colon adenocarcinomas compared to using either compound alone, even at higher doses [50].
Based on these prior studies, inhibiting ODC by DFMO may be used as a future agent for FAP suppression and progression into cancer. It is worth mentioning that troglitazone, an indirect inhibitor of ODC, induced apoptosis in an esophageal adenocarcinoma cell line but had no effect in an esophageal squamous cell carcinoma cell line [51]. Thus, the type of ODC inhibitor may have different effects on cancer chemoprophylaxis.
Although there are many studies on chemoprophylaxis agents, those about DFMO are still scarce. This systematic review represents the first comprehensive study on DFMO chemoprophylaxis use in GI neoplasms. Considering this, a variety of studies should be conducted on its effectiveness as a single agent or in combination with other chemopreventive agents. Furthermore, the benefit versus harm in treatment with these agents must be considered, along with its cost and long-term treatment associated side effects. For instance, some clinical trials using DFMO as a chemoprophylaxis resulted in a treatment-limiting toxicity, but lower effective doses did not result in ototoxicity [15, 52]. However, other side effects have not been reported or studied yet. Therefore, extensive studies should be made on DFMO before its use in chemoprophylaxis.
Beyond its role in chemoprophylaxis, DFMO could be considered as a post-cancer maintenance therapy to potentially prevent or delay the emergence of new driver mutations including TTN mutations, TP53, MUC16, and LRP1B or the progression of malignant residual disease.
| Conclusions | ▴Top |
DFMO could play a role in chemoprevention of luminal GI cancers as an affordable and nontoxic option, particularly when combined with NSAIDs. However, additional RCTs are needed, especially to evaluate its effectiveness as a standalone agent.
| Supplementary Material | ▴Top |
Suppl 1. Medline search strategy.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
There is no conflict of interest to declare by all authors.
Author Contributions
Conception and design: Ambar Godoy, Daniel A. Guifarro, and Daniela Montalvan-Sanchez. Administrative support: Fortunato S. Principe-Meneses, Adrian Riva-Moscoso, Leandro Sierra, Gloria Erazo, Carlos Avila, Mirian Ramirez-Rojas, and Roberto Giron. Provision, collection, and assembly of data: Leandro Sierra, Gloria Erazo, Carlos Avila, and Mirian Ramirez-Rojas. Literature review, manuscript draft, and revision of key components: all authors. Final approval of manuscript and agreement to be accountable for all aspects of the work: all authors.
Data Availability
The data validating the findings of this study can be obtained from the corresponding author upon reasonable request.
| References | ▴Top |
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Lloyd-Jones DM. USPSTF report on aspirin for primary prevention. JAMA Cardiol. 2022;7(7):667-669.
doi pubmed - Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181-193.
doi pubmed - Flossmann E, Rothwell PM, British Doctors Aspirin T, the UKTIAAT. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603-1613.
doi pubmed - Hahn E, Kraus S, Arber N. Role of cyclooxygenase-2 in pathogenesis and prevention of colorectal cancer. Dig Dis. 2010;28(4-5):585-589.
doi pubmed - Ng K, Meyerhardt JA, Chan AT, Sato K, Chan JA, Niedzwiecki D, Saltz LB, et al. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst. 2015;107(1):345.
doi pubmed - Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741-1750.
doi pubmed - Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. Int J Mol Sci. 2018;19(8):2332.
doi pubmed - McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018;379(16):1509-1518.
doi pubmed - Laukaitis CM, Gerner EW. DFMO: targeted risk reduction therapy for colorectal neoplasia. Best Pract Res Clin Gastroenterol. 2011;25(4-5):495-506.
doi pubmed - Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, Sierra JC, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene. 2015;34(26):3429-3440.
doi pubmed - Sinicrope FA, Broaddus R, Joshi N, Gerner E, Half E, Kirsch I, Lewin J, et al. Evaluation of difluoromethylornithine for the chemoprevention of Barrett's esophagus and mucosal dysplasia. Cancer Prev Res (Phila). 2011;4(6):829-839.
doi pubmed - Zell JA, McLaren CE, Chen WP, Thompson PA, Gerner EW, Meyskens FL. Ornithine decarboxylase-1 polymorphism, chemoprevention with eflornithine and sulindac, and outcomes among colorectal adenoma patients. J Natl Cancer Inst. 2010;102(19):1513-1516.
doi pubmed - Meyskens FL, Jr., Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5(5):945-951.
pubmed - Gerner EW, Meyskens FL, Jr., Goldschmid S, Lance P, Pelot D. Rationale for, and design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids. 2007;33(2):189-195.
doi pubmed - Gerner EW, Meyskens FL, Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781-792.
doi pubmed - https://www.covidence.org.
- Horn Y, Schechter PJ, Marton LJ. Phase I-II clinical trial with alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. Eur J Cancer Clin Oncol. 1987;23(8):1103-1107.
doi pubmed - Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase II trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep. 1986;70(7):843-845.
pubmed - Burke CA, Dekker E, Lynch P, Samadder NJ, Balaguer F, Huneburg R, Burn J, et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med. 2020;383(11):1028-1039.
doi pubmed - Lynch PM, Burke CA, Phillips R, Morris JS, Slack R, Wang X, Liu J, et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2016;65(2):286-295.
doi pubmed - Meyskens FL, Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila). 2008;1(1):32-38.
doi pubmed - Balaguer F, Stoffel EM, Burke CA, Dekker E, Samadder NJ, Van Cutsem E, Lynch PM, et al. Combination of sulindac and eflornithine delays the need for lower gastrointestinal surgery in patients with familial adenomatous polyposis: post hoc analysis of a randomized clinical trial. Dis Colon Rectum. 2022;65(4):536-545.
doi pubmed - Sinicrope FA, Velamala PR, Song L, Viggiano TR, Bruining DH, Rajan E, Gostout CJ, et al. Efficacy of difluoromethylornithine and aspirin for treatment of adenomas and aberrant crypt foci in patients with prior advanced colorectal neoplasms. Cancer Prev Res (Phila). 2019;12(11):821-830.
doi pubmed - Morgan DR, Dominguez RL, Norwood DA, Montalvan-Sanchez EE, Piazuelo MB, Hernandez-Marrero J, Gonzalez-Pons M, et al. Evaluation of the safety and efficacy of eflornithine (difluoromethylornithine, DFMO) in patients with gastric premalignant conditions in the high incidence areas of Latin America. Gastroenterology. 2024;166(Suppl 1):S267.
doi - Hall MJ. Updates in chemoprevention research for hereditary gastrointestinal and polyposis syndromes. Curr Treat Options Gastroenterol. 2021;19(1):30-46.
doi pubmed - Yang L, Wang Y, Hu S, Wang X. Eflornithine for chemoprevention in the high-risk population of colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. Front Oncol. 2023;13:1281844.
doi pubmed - Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30(1):3-21.
pubmed - Ratnasinghe D, Tangrea J, Roth MJ, Dawsey S, Hu N, Anver M, Wang QH, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res. 1999;19(1A):171-174.
pubmed - Morris CD, Armstrong GR, Bigley G, Green H, Attwood SE. Cyclooxygenase-2 expression in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96(4):990-996.
doi pubmed - Morgan GP, Williams JG. Inflammatory mediators in the oesophagus. Gut. 1994;35(3):297-298.
doi pubmed - Alexiou GA, Lianos GD, Ragos V, Galani V, Kyritsis AP. Difluoromethylornithine in cancer: new advances. Future Oncol. 2017;13(9):809-819.
doi pubmed - Garewal HS, Gerner EW, Sampliner RE, Roe D. Ornithine decarboxylase and polyamine levels in columnar upper gastrointestinal mucosae in patients with Barrett's esophagus. Cancer Res. 1988;48(11):3288-3291.
pubmed - Murray-Stewart T, Ferrari E, Xie Y, Yu F, Marton LJ, Oupicky D, Casero RA, Jr. Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047. PLoS One. 2017;12(4):e0175917.
doi pubmed - Soda K. The mechanisms by which polyamines accelerate tumor spread. J Exp Clin Cancer Res. 2011;30(1):95.
doi pubmed - Thomas TJ, Thomas T, John S, Hsu HC, Yang P, Keinanen TA, Hyvonen MT. Tamoxifen metabolite endoxifen interferes with the polyamine pathway in breast cancer. Amino Acids. 2016;48(10):2293-2302.
doi pubmed - Vainio H, Morgan G. NSAIDs, Barrett's oesophagus and adenocarcinoma prevention. Eur J Cancer Prev. 1997;6(2):200.
pubmed - Bakshi A, Cao Y, Orchard SG, Carr PR, Joshi AD, Manning AK, Buchanan DD, et al. Aspirin and the risk of colorectal cancer according to genetic susceptibility among older individuals. Cancer Prev Res (Phila). 2022;15(7):447-454.
doi pubmed - Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12(1):88-93.
doi pubmed - Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW, Jr. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53(6):1322-1327.
pubmed - Thun MJ, Namboodiri MM, Heath CW, Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325(23):1593-1596.
doi pubmed - Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124(1):47-56.
doi pubmed - Reddy BS, Rao CV. Colon cancer: a role for cyclo-oxygenase-2-specific nonsteroidal anti-inflammatory drugs. Drugs Aging. 2000;16(5):329-334.
doi pubmed - Hwang WY, Park WH, Suh DH, Kim K, Kim YB, No JH. Difluoromethylornithine induces apoptosis through regulation of AP-1 signaling via JNK phosphorylation in epithelial ovarian cancer. Int J Mol Sci. 2021;22(19):10255.
doi pubmed - Alhosin M, Razvi SSI, Sheikh RA, Khan JA, Zamzami MA, Choudhry H. Thymoquinone and difluoromethylornithine (DFMO) synergistically induce apoptosis of human acute T lymphoblastic leukemia Jurkat cells through the modulation of epigenetic pathways. Technol Cancer Res Treat. 2020;19:1533033820947489.
doi pubmed - Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;243(5):C212-221.
doi pubmed - Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48(4):759-774.
pubmed - Meyskens FL, Jr., Emerson SS, Pelot D, Meshkinpour H, Shassetz LR, Einspahr J, Alberts DS, et al. Dose de-escalation chemoprevention trial of alpha-difluoromethylornithine in patients with colon polyps. J Natl Cancer Inst. 1994;86(15):1122-1130.
doi pubmed - Reddy BS, Rao CV. Chemoprophylaxis of colon cancer. Curr Gastroenterol Rep. 2005;7(5):389-395.
doi pubmed - Takashima T, Fujiwara Y, Higuchi K, Arakawa T, Yano Y, Hasuma T, Otani S. PPAR-gamma ligands inhibit growth of human esophageal adenocarcinoma cells through induction of apoptosis, cell cycle arrest and reduction of ornithine decarboxylase activity. Int J Oncol. 2001;19(3):465-471.
pubmed - Schweitzer VG. Ototoxicity of chemotherapeutic agents. Otolaryngol Clin North Am. 1993;26(5):759-789.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.