| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 000, Number 000, August 2025, pages 000-000
Predictors of Development of Hepatocellular Carcinoma in Non-Cirrhotic Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis - A Retrospective Analysis of the National Inpatient Sample Database
Samyak Dhruva, d, Shravya Ginnaramb, Audrey Fonkamc, John Bogerc
aDepartment of Internal Medicine, Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA, USA
bDepartment of Gastroenterology and Hepatology, University of Nebraska Medical Center, Omaha, NE, USA
cDepartment of Gastroenterology and Hepatology, Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA, USA
dCorresponding Author: Samyak Dhruv, Department of Internal Medicine, Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA, USA
Manuscript submitted June 10, 2025, accepted July 21, 2025, published online August 7, 2025
Short title: HCC Risk in Non-Cirrhotic MASLD/MASH Patients
doi: https://doi.org/10.14740/gr2053
| Abstract | ▴Top |
Background: One-quarter of the world population is thought to have metabolic dysfunction-associated steatotic liver disease (MASLD). The incidence of metabolic dysfunction-associated steatohepatitis (MASH) and MASLD is rapidly increasing due to the ongoing global epidemic of type 2 diabetes mellitus and obesity. Hepatitis B and C have declined in incidence due to advances in prevention and treatment, yet the overall burden of hepatocellular carcinoma (HCC) continues to rise, largely driven by the growing prevalence of MASLD/MASH. MASLD/MASH is now the fastest-growing etiology of HCC in the USA, France and the UK, with an estimated annual incidence of HCC ranging from 0.5% to 2.6% in patients with MASH cirrhosis. The incidence of HCC among patients with non-cirrhotic MASLD/MASH is lower, approximately 0.1% to 1.3%. There are no screening guidelines currently for HCC in non-cirrhotic MASLD/MASH patients. Our study highlights the dire need to develop HCC predictive strategies and algorithm in this non-cirrhotic population and to move away from a cirrhotic-centered approach but rather use risk-based models. We have identified predictors of development of HCC in this patient population that can be used to develop risk-based HCC screening guidelines and models in a non-cirrhotic population with MASLD/MASH.
Methods: Nationwide Inpatient Sample (NIS) database from 2016 to 2019 was used in this analysis. Chi-square test and t-test and were used to establish association between two variables. The significant variables were included in the logistic regression model to identify independent association between variables.
Results: From the NIS database, 1,326,230 non-cirrhotic MASLD/MASH patients were identified. The mean age was 53.75 years; 52% were female. Older age (P < 0.0001), female gender (adjusted odds ratio (AOR) = 1.303, P < 0.001), and Asian race (AOR = 1.135, P = 0.01) were associated with increased HCC risk. Anemia, leukopenia, hyponatremia, and hypoalbuminemia were independent predictors (all P < 0.001). Benign liver lesions such as focal nodular hyperplasia (AOR = 1.269) and hemangiomas (1.475), as well as infections like cholangitis (3.093) and liver abscess (2.073), were linked to higher risk. Autoimmune diseases, including rheumatoid arthritis (0.679) and systemic lupus erythematosus (SLE, 0.456), were associated with decreased HCC risk (P < 0.001).
Conclusions: This study provides compelling evidence that HCC can develop in non-cirrhotic MASLD/MASH patients. These findings highlight an urgent need to shift from a cirrhosis-centric approach to a more comprehensive, risk-based HCC screening model - especially given that MASLD/MASH is now the most common and fastest-growing etiology of HCC around the globe. This study can potentially help develop those screening guidelines to prevent the development or early detection of HCC in non-cirrhotic MASLD/MASH patients.
Keywords: Liver cirrhosis; Hepatocellular carcinoma; Non-cirrhotic; Non-alcoholic fatty liver disease; Non-alcoholic steatohepatitis; Metabolic dysfunction-associated steatotic liver disease; Metabolic dysfunction-associated steatohepatitis
| Introduction | ▴Top |
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis which is confirmed through imaging or histology. There should also be an absence of secondary causes of hepatic fat accumulation like heavy alcohol consumption, long-term use of steatogenic medications and other chronic liver diseases [1].
In recent years, there has been an introduction of the term metabolic dysfunction-associated steatotic liver disease (MASLD) which removes the need to exclude other liver diseases from NAFLD definition and instead focuses on inclusive diagnostic criteria [2, 3]. MASLD is diagnosed based on evidence of hepatic steatosis (via histology, imaging, or blood biomarkers) along with one of the following: obesity, type 2 diabetes mellitus (T2DM) and/or evidence of metabolic dysregulation [2]. The term NAFLD has been mostly replaced by MASLD to focus more on the metabolic dysfunction related to underlying conditions such as obesity, type 2 diabetes, and metabolic syndrome. MASLD is a more encompassing term that will include more patients than NAFLD with broad metabolic profiles.
MASLD is one of the most prevalent causes of chronic liver disease globally, affecting over 25% of the world population. Among these patients, up to 25% may develop metabolic dysfunction-associated steatohepatitis (MASH), with or without liver fibrosis [4]. MASH, particularly when accompanied by fibrosis, represents progression of disease, associated with significant morbidity and mortality due to complications such as cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).
While cirrhosis is a major risk factor for HCC, new studies have suggested that in MASLD-related HCC, up to 50% of patients did not have clinical or histological evidence of cirrhosis at diagnosis [5]. Studies have also suggested that patients with non-cirrhotic MASLD have a 2.5 times increased risk of developing HCC compared to those with other etiologies of chronic liver disease without cirrhosis [6]. MASLD/MASH is now the fastest-growing etiology of HCC in the USA, France and the UK, with an estimated annual incidence of HCC ranging from 0.5% to 2.6% in patients with MASH cirrhosis. The incidence of HCC among patients with non-cirrhotic MASLD/MASH is lower, approximately 0.1% to 1.3% [7].
| Materials and Methods | ▴Top |
The Nationwide Inpatient Sample (NIS) is an administrative database and part of the Healthcare Cost and Utilization Project (HCUP). It is the largest publicly available all-payer inpatient healthcare database designed to produce US regional and national estimates of inpatient utilization, access, cost, quality, and outcomes. Unweighted, it contains data from more than 7 million hospital stays each year. Weighted, it estimates more than 35 million hospitalizations nationally.
All adult hospitalizations with an International Classification of Disease, 10th Revision (ICD-10) diagnosis code related to NAFLD and non-alcoholic steatohepatitis (NASH) were identified from the NIS database for the years 2016 to 2019. From these, hospitalizations that had a diagnosis code of cirrhosis were removed. After removing cirrhotic patients, patients with hepatitis B, hepatitis C, hemochromatosis, alpha-1 antitrypsin deficiency, Wilson’s disease, hyper-citrullinemia, hepatic porphyria and glycogen storage diseases were also removed as potential confounders (Fig. 1). All these confounders had at least one case of HCC in the literature that had developed in a non-cirrhotic patient. All hospitalizations were stratified into two categories 1) MASLD/MASH cases without HCC; and 2) MASLD/MASH cases with HCC. Furthermore, ICD-10 diagnosis codes were used to identify the presence of comorbidities including diabetes mellitus (DM) (type 1 and 2), hypertension (HTN), hyperlipidemia (HLD), atrial fibrillation, and other comorbid conditions. ICD-10 codes were also used to identify benign liver lesions and autoimmune disorders (Table 1).
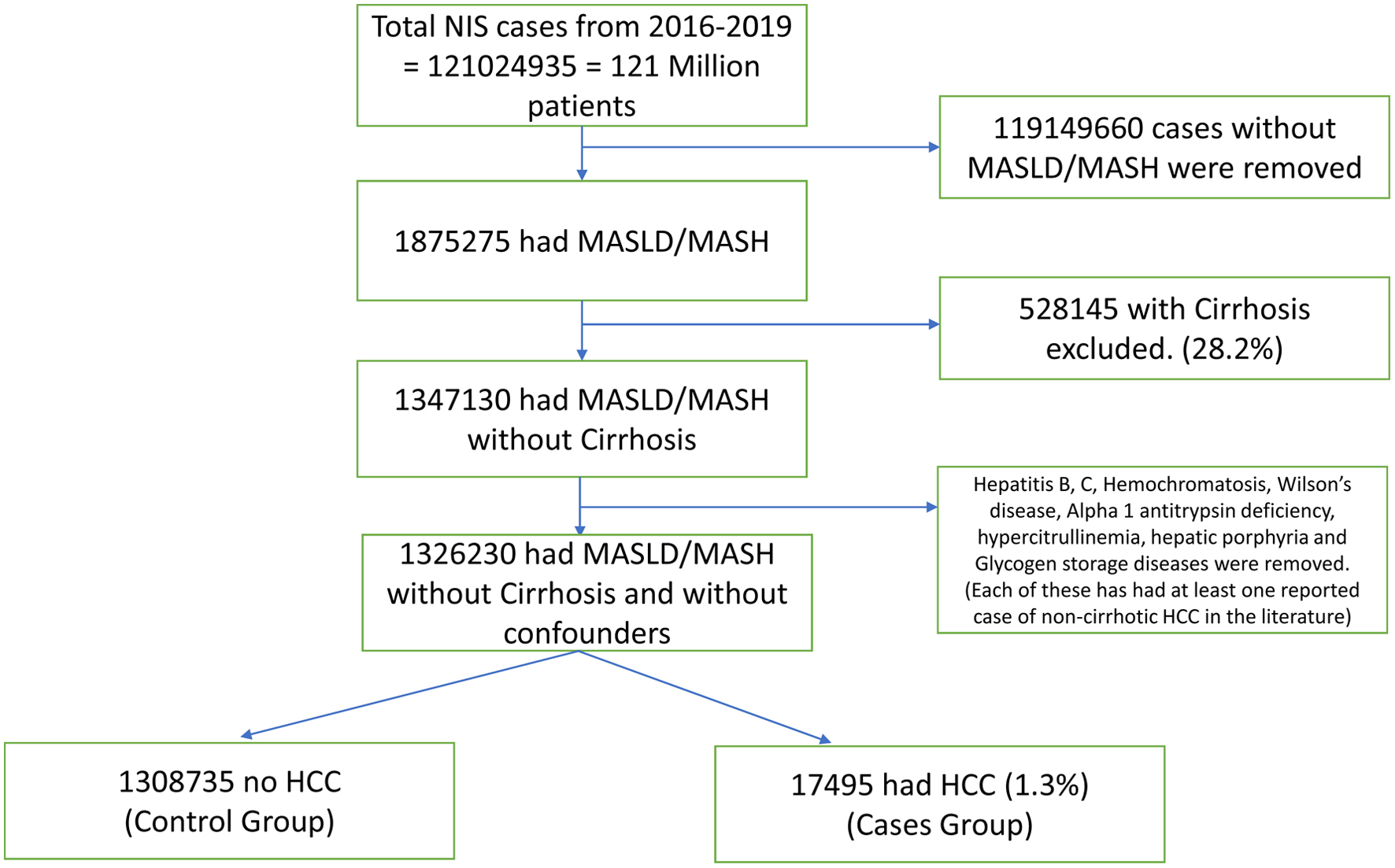 Click for large image | Figure 1. Consort diagram for non-cirrhotic HCC. HCC: hepatocellular carcinoma; MASLD: metabolic dysfunction-associated steatotic liver disease; MASH: metabolic dysfunction-associated steatohepatitis. |
 Click to view | Table 1. Clinical Characteristics and Outcomes of Non-Cirrhotic Patients With MASLD/MASH (Univariate Analysis) |
The primary outcome of the study was identifying HCC cases in non-cirrhotic MASLD/MASH population. Secondary outcomes included the role of age and sex in these HCC cases.
Chi-square tests were utilized to assess the association between qualitative variables. Mann Whitney-U test and t-tests were utilized to compare the mean as appropriate. Bivariate analysis was conducted to identify predictors of development of HCC in non-cirrhotic patients with MASLD/MASH. The predictors significant in bivariate analysis (Table 2) were included in the multivariate model to establish independent association of development of HCC in these patients (Table 3). All the analyses were performed using IBM SPSS Statistics 28.0.0.0 (International Business Machines Statistical Package for Social Sciences Version 28.0.0.0 (Armonk, NY, USA).
 Click to view | Table 2. Predictors of Development of HCC in Non-Cirrhotic Patients With MASLD/MASH (Bivariate Analysis) |
 Click to view | Table 3. Odds of Development of HCC in Non-Cirrhotic MASLD/MASH Patients in Bivariate and Multivariate Analysis (Multivariate Logistic Regression Analysis) |
The data used for this study were deidentified patient data and were thus exempt from the Institutional Review Board process. This study was conducted in compliance with all the applicable institutional ethical guidelines for care and welfare.
| Results | ▴Top |
A total of 1,326,230 cases of MASLD/MASH were identified. Average age of the population was 53.75 years, and females comprised 52% of the cohort. Older age (P < 0.0001) and female gender (AOR = 1.303, P <0.001) were associated with higher risk of development of HCC. Additionally, the Asian population (AOR = 1.135, P = 0.01) had the highest risk followed by White Americans. Anemia (1.247), leukopenia (1.360), hyponatremia (1.389), and hypoalbuminemia (2.041) were independently associated with higher risk of HCC (P < 0.001). Benign liver lesions like focal nodular hyperplasia (1.269) and hepatic hemangiomas (1.475) and hepatobiliary infections like cholangitis (3.093) and liver abscesses (2.073) were also associated with higher risk of HCC in non-cirrhotic MASLD/MASH patient population (P < 0.001). Contrary to other comorbid conditions and patient factors, autoimmune disorders like rheumatoid arthritis (0.679) and systemic lupus erythematosus (SLE) (0.456) were associated with lower risk of development of HCC in this patient population (P < 0.001).
| Discussion | ▴Top |
The etiology of HCC is shifting, with MASLD and MASH becoming increasingly prominent. While hepatitis B and C were once considered the most common causes of HCC, their incidence has declined due to vaccination and potent antiviral therapies. However, the overall incidence of HCC continues to rise, largely driven by the rapidly increasing incidence of MASLD/MASH-related HCC across the globe [8]. In Shanghai, age-standardized HCC rates in males decreased from 33.38 to 17.34 per 100,000, and in females from 11.65 to 5.60 between 1973 and 2014. At the same time, the global prevalence of MASLD rose from 26% in 2005 to 38% in 2016. MASH is now the fastest-growing cause of HCC among patients listed for liver transplantation in the United States and the cause of death globally (+39%) [7, 8].
This shift challenges the traditional understanding of HCC risk, where cirrhosis was once considered a prerequisite. Our study identified a 1.3% prevalence of HCC in non-cirrhotic MASLD/MASH patients (17,495 out of 1,326,230). Previous studies have suggested that up to 35% of MASLD-related and up to 54% of MASH-related HCC cases occur in patients without cirrhosis, suggesting that current surveillance guidelines may be missing a significant proportion of those patients without cirrhosis who are at risk of development of HCC [9-11].
Certain demographic and clinical factors increase the risk of HCC in this population. Age emerged as a strong predictor, with a mean age of 62 years in HCC compared to 53 years in non-HCC patients, consistent with prior research [12]. Interestingly, female sex accounted for 58.4% (OR = 1.301, P < 0.001) of cases, which contrasts with the conventional understanding of male predominance in all cause HCC [13]. The biological basis for reported male predominance in HCC is unclear but may be driven by estrogen’s protective effect, lower adiponectin levels, and upregulation of oncogenic pathways such as SRY, CCRK, Foxa, miR-216a, and miR-22 in men.
Although estrogen is thought to offer protective effects against MASLD/MASH in premenopausal women, several studies have demonstrated a substantial rise in the incidence and severity of MASLD/MASH among postmenopausal women compared to men [14]. Given that the mean age of patients with HCC in our cohort was 62 years, this age-related hormonal shift may partly explain the higher observed risk of MASLD/MASH-related HCC in the female population. Lifestyle factors such as alcohol use, smoking, and higher hepatitis B and C virus (HBV/HCV) prevalence may further contribute. In postmenopausal women, this pattern may reverse due to the loss of estrogen and rising metabolic risk implicated in increased hepatic inflammation, lipid accumulation, and fibrosis progression, which could predispose women to HCC [15]. Additionally, whole-exome sequencing studies have identified polymorphisms in the ALPK2 and Wnt/β-catenin signaling pathways that are more frequently expressed in female MASLD patients, potentially accelerating tumorigenesis [16]. However, females have been observed to have better survival outcomes compared to males post locoregional therapy or resection, with smaller tumor size at diagnosis and low risk for portal vein thrombosis or renal dysfunction [11, 17].
Race and ethnicity also played a significant role in HCC risk in our study. Among non-cirrhotic MASLD/MASH patients, Asian patients had the highest likelihood of developing HCC, with an odds ratio (OR) of 1.875 (P < 0.001). This was followed by White patients, who showed a modest but significant increased risk (OR = 1.052, P = 0.002). In contrast, African American and Hispanic patients had lower odds of developing HCC compared to White patients, with OR of 0.781 (P < 0.001) and 0.849 (P < 0.001), respectively.
Several metabolic and laboratory markers also showed strong associations with HCC risk. DM was a significant predictor, supporting evidence that hyperinsulinemia, chronic inflammation, and hepatic lipotoxicity contribute to carcinogenesis even without cirrhosis [18]. Hypoalbuminemia, anemia, hyponatremia, and leukopenia were also associated with HCC risk, though their roles remain less clearly defined. Beyond metabolic factors, biliary and infectious processes appeared to play an important role in liver cancer development. Patients with a history of cholangitis had a markedly higher risk of HCC, with an OR > 4.0. Liver abscess was also independently associated with HCC, raising the possibility that hepatic inflammation and microbiome alterations contribute to malignant transformation [19]. Though not typically associated with development into HCC, benign liver lesions such as focal nodular hyperplasia (OR = 1.543, P < 0.001) and liver hemangiomas (OR = 1.398, P = 0.003) were found to coexist more commonly in patients who developed HCC. While causality cannot be established, this finding may suggest vulnerability from pre-existing hepatic structural abnormalities, early imaging misclassification or simply more imaging in these patient populations leading to earlier diagnosis of HCC. To our surprise, autoimmune disorders like rheumatoid arthritis and SLE were protective against development of HCC. This interaction may be very complex and needs to be further investigated with prospective studies and randomized clinical trials. Zhang et al have shown decreased incidence of HCC in rheumatoid arthritis patients [20]. Steroids like dexamethasone have also shown anti-tumorigenic properties in rat models [21]. Steroids are one of the most common drugs used in these autoimmune diseases. Also in our study, liver abscess and cholangitis had increased risk of HCC, suggesting a potential role of chronic inflammation in the development of HCC, which is common in autoimmune diseases as well.
This study has significant clinical implications, particularly for HCC screening and surveillance. Current guidelines focus almost exclusively on cirrhotic patients, leaving a substantial proportion of non-cirrhotic MASLD/MASH patients at risk but undetected [22]. Given the increasing burden of MASLD and its potential to progress to liver cancer even in the absence of fibrosis, it is critical to reconsider how HCC risk is assessed in these patients. Noninvasive biomarkers, advanced imaging modalities, and risk stratification tools incorporating metabolic and inflammatory markers may help refine early detection efforts [23].
There are some limitations to our study. The retrospective nature of the study and use of ICD-10 codes rely a lot on physician documentation and coding. The casual association of the significant predictors and HCC cannot be established. It is possible that a subset of patients may have had underlying liver cirrhosis that was not clinically recognized at the time of coding, which could have influenced certain outcomes. Our study has several significant strengths on the other end. We have used a large sample size that increases the external validity of the study. We have also used several predictors and variables with the exclusion of significant confounders making our study reliable.
Conclusions
In conclusion, this study provides evidence that HCC can develop in non-cirrhotic MASLD/MASH patients, particularly those with older age, female sex and patients with diabetes, hypoalbuminemia, and a history of biliary infections. These findings highlight an urgent need to shift from a cirrhosis-centric approach to a more comprehensive, risk-based HCC screening model, especially since MASLD/MASH is now the most common and fastest-growing etiology of HCC around the globe. Future research should focus on refining algorithms, integrating sex-specific risk factors, and developing surveillance strategies to ensure early detection and improved outcomes in this vulnerable population to curb the global epidemic of MASLD/MASH and thus HCC. While noninvasive tools such as the MELD and Child-Pugh scores are widely used to predict outcomes in patients with liver cirrhosis [24], there remains a lack of validated predictive models for patients without cirrhosis. Our study identifies key clinical and laboratory predictors that could serve as the foundation for developing a risk-based model to estimate HCC risk in non-cirrhotic MASLD/MASH patients, thereby enabling timely surveillance, facilitating early detection, and potentially preventing progression to advanced HCC.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Our study uses deidentified NIS database and does not need patient consent.
Author Contributions
Dr. Samyak Dhruv is the main author who came up with this unique idea; he did the statistical analysis including bivariate and multivariate analysis with logistic regression and made initial manuscript draft. He also did the editing and finalized the draft for the publication. Dr. Shravya Ginnaram helped with writing manuscript. Dr. Audrey Fonkam did the review from a hepatological standpoint. Dr. John Boger is the main attending on our paper. He has provided his valuable opinion in creating the study design, he also helped with statistical analysis and final review of the draft. He remains expert on our study.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
HCC: hepatocellular carcinoma; MASLD: metabolic dysfunction-associated steatotic liver disease; MASH: metabolic dysfunction-associated steatohepatitis; NIS: National Inpatient Sample; ICD-10: International Classification of Disease, 10th Revision; HCUP: Healthcare Cost and Utilization Project; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis
| References | ▴Top |
- Guo X, Yin X, Liu Z, Wang J. Non-Alcoholic Fatty Liver Disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int J Mol Sci. 2022;23(24):15489.
doi pubmed - Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): a State-of-the-Art review. J Obes Metab Syndr. 2023;32(3):197-213.
doi pubmed - Rinella ME, Sookoian S. From NAFLD to MASLD: updated naming and diagnosis criteria for fatty liver disease. J Lipid Res. 2024;65(1):100485.
doi pubmed - Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11-20.
doi pubmed - Foerster F, Gairing SJ, Muller L, Galle PR. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J Hepatol. 2022;76(2):446-457.
doi pubmed - Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75(6):1476-1484.
doi pubmed - Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34(7):969-977.e962.
doi pubmed - Koshy A. Evolving Global Etiology of Hepatocellular Carcinoma (HCC): insights and trends for 2024. J Clin Exp Hepatol. 2025;15(1):102406.
doi pubmed - Leyh C, Coombes JD, Schmidt HH, Canbay A, Manka PP, Best J. MASLD-related HCC-update on pathogenesis and current treatment options. J Pers Med. 2024;14(4):370.
doi pubmed - Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63(3):827-838.
doi pubmed - Polyzos SA, Chrysavgis L, Vachliotis ID, Chartampilas E, Cholongitas E. Nonalcoholic fatty liver disease and hepatocellular carcinoma:Insights in epidemiology, pathogenesis, imaging, prevention and therapy. Semin Cancer Biol. 2023;93:20-35.
doi pubmed - Vitellius C, Desjonqueres E, Lequoy M, Amaddeo G, Fouchard I, N'Kontchou G, Canivet CM, et al. MASLD-related HCC: Multicenter study comparing patients with and without cirrhosis. JHEP Rep. 2024;6(10):101160.
doi pubmed - Chen YT, Chen TI, Yang TH, Yin SC, Lu SN, Liu XR, Gao YZ, et al. Long-term risks of cirrhosis and hepatocellular carcinoma across steatotic liver disease subtypes. Am J Gastroenterol. 2024;119(11):2241-2250.
doi pubmed - Meda C, Dolce A, Della Torre S. Metabolic dysfunction-associated steatotic liver disease across women's reproductive lifespan and issues. Clin Mol Hepatol. 2025;31(1):327-332.
doi pubmed - Greten TF. Gender disparity in HCC: Is it the fat and not the sex? J Exp Med. 2019;216(5):1014-1015.
doi pubmed - Wei J, Wu BJ, Daoud SS. Whole-Exome Sequencing (WES) Reveals Novel Sex-Specific Gene Variants in Non-Alcoholic Steatohepatitis (MASH). Genes (Basel). 2024;15(3):357.
doi pubmed - Huang J, Wei S, Tang Y, Zhang Q, Luo H, Tang Z, Tang Y, et al. Sex differences in the impact of metabolic dysfunction-associated fatty liver disease on the of patients with hepatocellular carcinoma after radical resection. J Cancer. 2023;14(7):1107-1116.
doi pubmed - Meroni M, Longo M, Dongiovanni P. Cardiometabolic risk factors in MASLD patients with HCC: the other side of the coin. Front Endocrinol (Lausanne). 2024;15:1411706.
doi pubmed - Chen J, Lu L, Qu C, A G, Deng F, Cai M, Chen W, et al. Body mass index, as a novel predictor of hepatocellular carcinoma patients treated with Anti-PD-1 immunotherapy. Front Med (Lausanne). 2022;9:981001.
doi pubmed - Zhang Y, Zhang Y, He P, Ge F, Huo Z, Qiao G. The genetic liability to rheumatoid arthritis may decrease hepatocellular carcinoma risk in East Asian population: a Mendelian randomization study. Arthritis Res Ther. 2023;25(1):49.
doi pubmed - Motafeghi F, Mortazavi P, Ghassemi-Barghi N, Zahedi M, Shokrzadeh M. Dexamethasone as an anti-cancer or hepatotoxic. Toxicol Mech Methods. 2023;33(2):161-171.
doi pubmed - Ma Y, Wang J, Xiao W, Fan X. A review of MASLD-related hepatocellular carcinoma: progress in pathogenesis, early detection, and therapeutic interventions. Front Med (Lausanne). 2024;11:1410668.
doi pubmed - Yun B, Park H, Ahn SH, Oh J, Kim BK, Yoon JH. Liver cancer risk across metabolic dysfunction-associated steatotic liver disease and/or alcohol: a nationwide study. Am J Gastroenterol. 2025;120(2):410-419.
doi pubmed - Scarlata GGM, Ismaiel A, Gambardella ML, Leucuta DC, Luzza F, Dumitrascu DL, Abenavoli L. Use of non-invasive biomarkers and clinical scores to predict the complications of liver cirrhosis: a bicentric experience. Medicina (Kaunas). 2024;60(11):1854.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.