| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 5, October 2025, pages 254-261
Histologic Assessment in Ulcerative Colitis: A Survey of Pathologists’ Practices and Perspectives
Krithika D. Shenoya, Jiannan Lia, Adam L. Bootha, Xiuli Liua, b
aDepartment of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri, USA
bCorresponding Author: Xiuli Liu, Department of Pathology and Immunology, Washington University in St. Louis School of Medicine, St. Louis, MO 63110, USA
Manuscript submitted July 17, 2025, accepted September 25, 2025, published online October 9, 2025
Short title: Histological Assessment in Ulcerative Colitis
doi: https://doi.org/10.14740/gr2065
| Abstract | ▴Top |
Background: Histologic remission is increasingly recognized as an important endpoint in ulcerative colitis (UC) management. Consensus guidelines on adopting histologic scoring systems in clinical practice are lacking in the United States. This study aimed to assess the knowledge, attitudes, and practices of pathologists, primarily located in North America, regarding histologic evaluation in UC.
Methods: This study surveyed a group of pathologists who have completed postdoctoral medical training with demonstrated interest and involvement in the field of gastrointestinal pathology to evaluate their knowledge, practices, and perspectives on histologic assessment using standardized scoring systems in clinical practice in UC patients. The survey was hosted on an online platform, and responses were recorded anonymously.
Results: A total of 57 responses were included in the analysis. Nearly two-thirds of pathologists acknowledged a lack of familiarity with the criteria for histologic remission as defined by the Nancy Index (NI), Robarts Histopathology Index (RHI), and Geboes score (GS). A majority (37/57; 65%) of pathologists did not support routine inclusion of a histologic score in pathology reports. The remaining 20/57 (35%) pathologists advocated for the incorporation of a standardized index, with the NI favored by 10/20 (50%) followed by the GS (n = 3; 15%) and the IBD-Distribution, Chronicity and Activity score (n = 3; 15%). Nearly a half (27/57; 47%) of the respondents acknowledged a favorable role for artificial intelligence in this setting.
Conclusions: The current survey highlights the need for collaborative efforts among pathologists, gastroenterologists, and professional societies to establish a consensus guideline for routine histologic assessment in UC. Additional guidance from professional societies and research are required to integrate artificial intelligence-driven approaches into routine clinical practice.
Keywords: Artificial intelligence; Geboes score; Histologic remission; Nancy Index; Robarts Histopathology Index; Survey; Ulcerative colitis
| Introduction | ▴Top |
As treatments for ulcerative colitis (UC) continue to advance, a “treat-to-target” approach has gained prominence, focusing on normalization objective disease measures. Emerging evidence suggests that histologic remission is a more reliable predictor of clinical relapse, corticosteroid use, and hospitalization compared to clinical and endoscopic findings [1-3]. This understanding has driven efforts to standardize histologic assessment in clinical trials, a critical step towards documenting treatment effects and informing therapeutic decisions in UC [4].
While clinical and endoscopic remission remain primary treatment goals in clinical practice, the International Organization for the Study of Inflammatory Bowel Diseases has recently recognized histologic remission as an adjunctive target in UC [5]. Unlike endoscopic reports, which largely incorporate a standardized endoscopic measure such as the Mayo Endoscopic Score or the Ulcerative Colitis Endoscopic Index of Severity, histologic reporting remains mostly descriptive and nonstandardized [6-8]. Despite the lack of a universally accepted definition for histologic remission in UC, there is a general consensus among inflammatory bowel disease (IBD) experts that features such as a neutrophil-free mucosa are essential components [4]. However, only a few of the described histologic scoring systems, of which there are over 30, have undergone some form of index validation. Among these, the Nancy Index (NI) and the Robarts Histopathology Index (RHI) have emerged as the most validated for their construct, content, and criteria, and have been tested for reliability between raters albeit in a research setting [9-12]. Consequently, the 2020 revised European Crohn’s and Colitis Organization guidelines recommend using the NI or the RHI for histologic evaluation of treated UC patients in a randomized controlled trial setting, with the NI additionally endorsed for use in observational studies and clinical practice [13]. However, the acceptance of these indices in the real world remains low.
Two recent surveys investigating the real-world use of histologic indices in UC highlighted the underutilization of histologic indices in this setting, yet both had limited representation from pathologists in the United States [6, 7]. To address this gap, we conducted a survey targeting pathologists in the United States and Canada to explore current knowledge, attitudes, and practices regarding histologic assessment in UC.
| Materials and Methods | ▴Top |
A 22-question survey was conducted to assess current knowledge, attitudes, and practices regarding the use of histologic indices for UC in clinical practice (Supplementary Material 1, gr.elmerpub.com). The survey was designed and administered via an online platform (QualtricsXM, Provo, Utah).
The invitations of this survey were distributed to 389 pathologists through the personal network of professional societies based in the United States of America or Canada. All responses were collected anonymously.
The survey consisted of two sections. The first section included nine questions related to respondent demographics and practice characteristics. The second section included 13 questions evaluating participants’ knowledge and attitudes toward standardized histologic indices in UC. All questions were mandatory for survey completion and inclusion in the final analysis. Knowledge scores were calculated based on responses to questions 10-18, which assessed participants’ familiarity with histologic indices, criteria for histologic remission, and key features of UC biopsy evaluation (see Supplementary Material 1 (gr.elmerpub.com) for point breakdown). The total knowledge score for each participant was calculated by summing the points across all items, resulting in a possible range of 0 - 23, with higher scores indicating greater knowledge. For the purpose of scoring, histologic remission was defined as a Geboes score (GS) ≤ 2.0, RHI ≤ 3, and NI grade < 2 [4, 13].
Statistical analysis
Data analysis was performed using the Stats iQ tool within the QualtricsXM platform. Descriptive statistics, including frequencies, percentages, and median values were calculated. Statistical differences were determined using Chi-square test for categorical variables. One-way analysis of variance (ANOVA) was applied to examine the relationship between knowledge scores and categorical variables. A P < 0.05 was considered statistically significant.
Institutional Review Board Approval is not applicable as this study does not involve any human or animal subjects or using any clinical or experimental data. Ethical compliance with human/animal study is not applicable as this study does not involve any human or animal subjects.
| Results | ▴Top |
Eighty-eight responses among 389 invitations were recorded with 57 complete surveys. The remaining 31 out of 88 respondents only completed the demographic section of the survey.
Demographic and practice characteristics of respondents
The majority of respondents were pathologists based in the United States of America or Canada, 34 (59.6%) of whom worked at university-affiliated academic centers and 36 (63.2%) had been in practice for > 10 years. Thirty-five pathologists (61.4%) reported reviewing > 10 cases/set of colonic biopsies from UC patients per week. Only 10 (17.5%) pathologists indicated that gastroenterologists at their institution requested histologic scoring to be reported, either at initial diagnosis (n = 2; 1.8%) or during therapeutic monitoring (n = 8; 14.0%). Among these, the NI was the most commonly reported scoring system, either alone or in conjunction with another histologic score, used by eight respondents, of whom four practiced in the United States (Fig. 1). Three (15%) respondents used GS. Of note, three (15%) respondents specified that they used a recently proposed histological index for IBD called “DCA score” which evaluates distribution, chronicity, and activity [14]. Additional demographic and practice characteristics are summarized in Table 1.
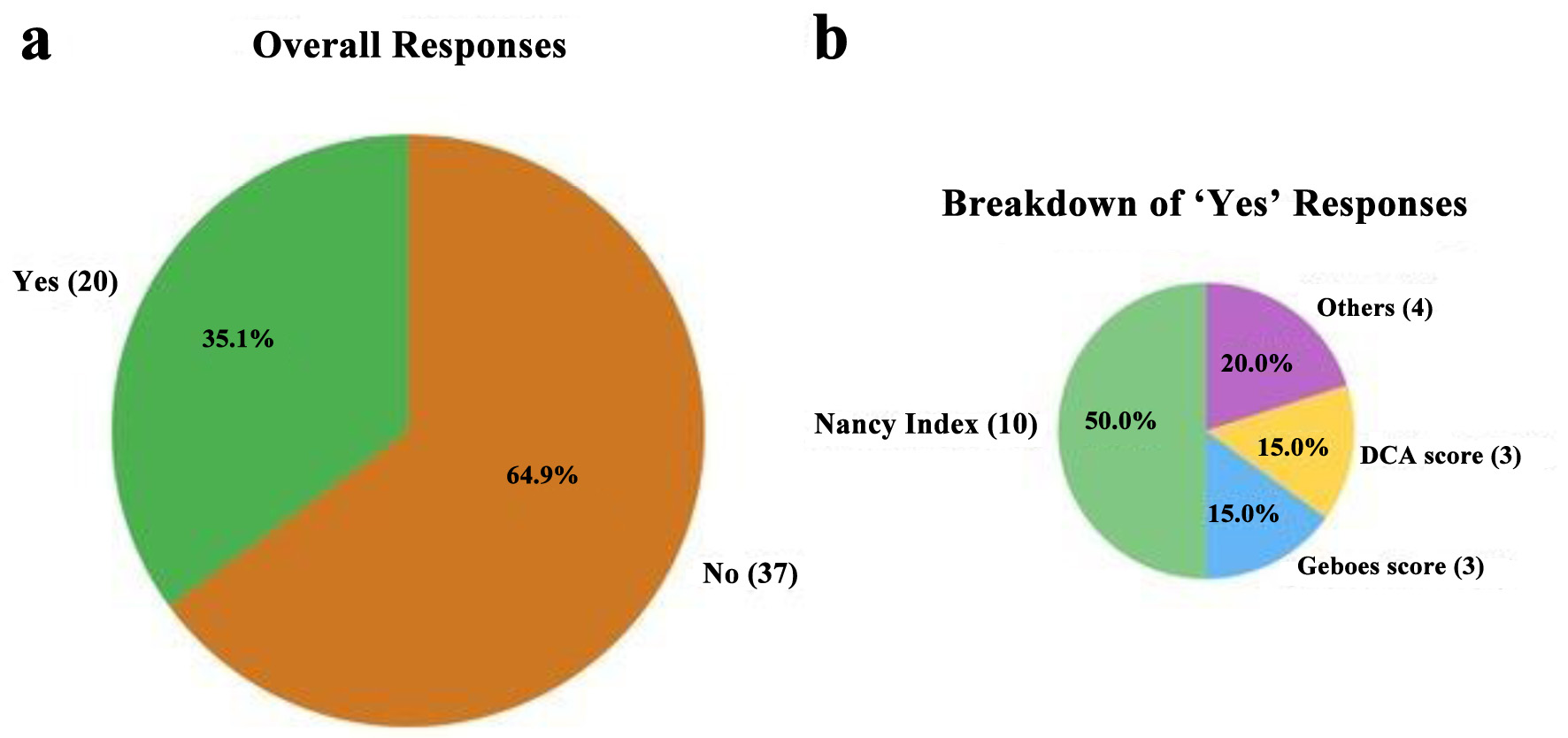 Click for large image | Figure 1. Survey responses regarding the use of standardized histologic indices for ulcerative colitis histologic activity assessment in routine practice. (a) Proportion of respondents reporting the use of any standard histologic index in routine pathology reporting (question 20, Supplementary Material 1, gr.elmerpub.com). (b) Distribution of specific histologic indices used among those who answered “yes” to panel (a). |
 Click to view | Table 1. Demographics and Practice Characteristics of the Survey Participants (N = 57) |
Knowledge of histologic indices and scoring systems in UC
The median knowledge score among survey participants was 10 out of 23 (range: 3 - 18). There were no statistically significant differences in knowledge scores between pathologists working at academic centers and those working in other practice settings (median score: 11 vs. 9; P = 0.26), nor between fellowship-trained gastrointestinal pathologists and those without gastrointestinal pathology fellowship training (median score: 10 vs. 11; P = 0.42).
Most respondents agreed that histologic activity in UC patients in endoscopic remission is associated with an increased risk of clinical relapse (n = 39; 68.4%) and neoplasia (n = 35; 61.4%). However, when asked about the role of standardized histologic indices in clinical practice, nearly half of the pathologists (n = 26; 45.6%) indicated that their role is not yet established. When queried about the specific definitions of histologic remission according to the NI, GS, and RHI, most pathologists reported unfamiliarity with these criteria (Fig. 2). Additionally, knowledge of common histologic features of IBD was assessed using series of statements rated on a five-point Likert scale from “strongly agree” to “strongly disagree.” The distribution of responses is summarized in Figure 3. When asked about the application of artificial intelligence (AI), most pathologists (n = 49; 86%) reported being unaware of any existing tools designed to aid histologic scoring in UC.
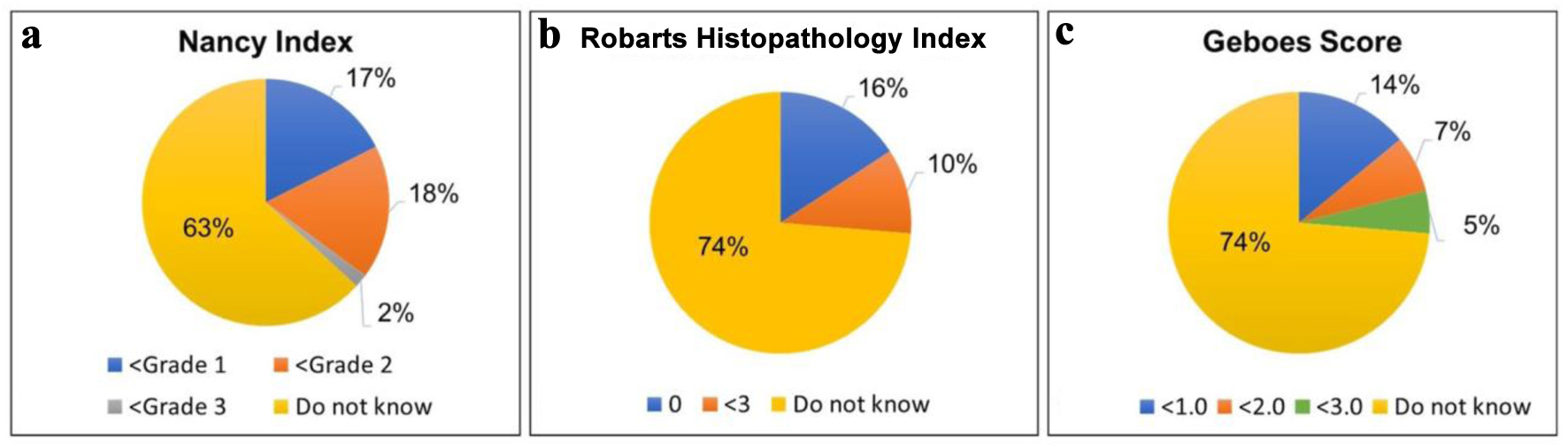 Click for large image | Figure 2. Distribution of responses among surveyed pathologists (N = 57) regarding the score thresholds they associate with histologic remission for three common histologic indices used in the assessment of colonic biopsies from ulcerative colitis patients: (a) Nancy Index, (b) Robarts Histopathology Index, and (c) Geboes Score. The “do not know” represents the percentage of respondents unfamiliar with the appropriate threshold for histologic remission for each index/scoring system. |
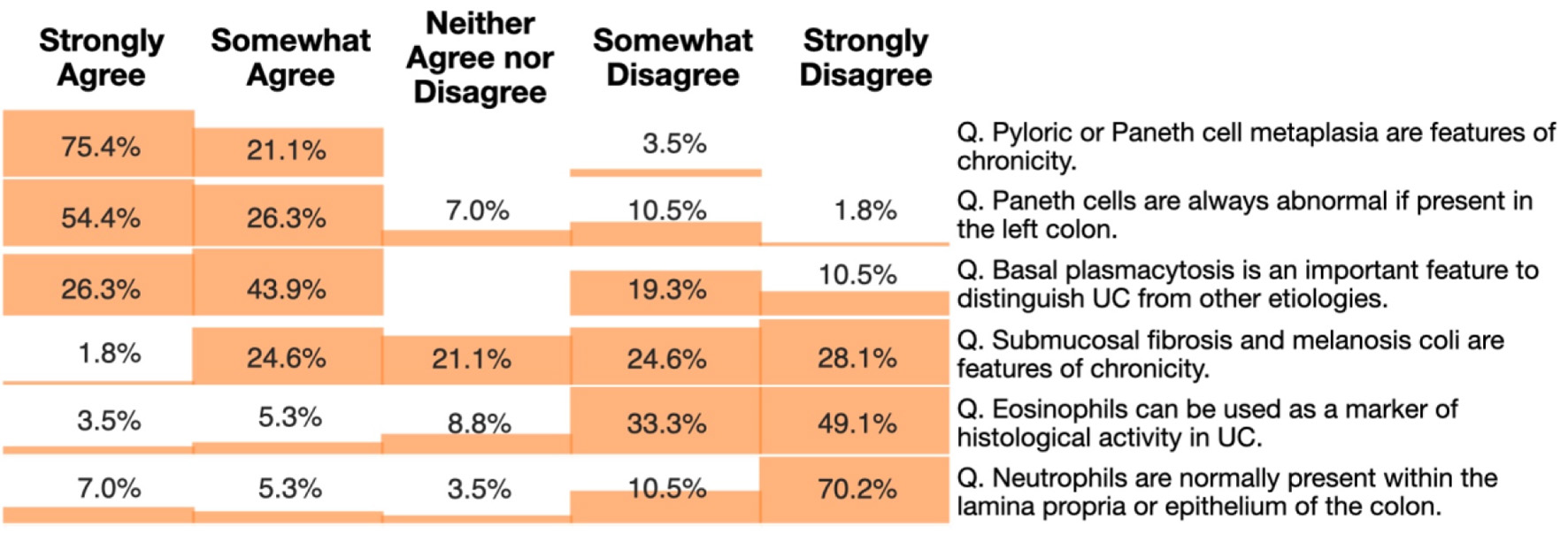 Click for large image | Figure 3. Heatmap showing pathologists’ responses to knowledge assessment questions on histologic features of inflammatory bowel disease. Participants rated their level of agreement with each statement using a five-point Likert scale: strongly agree, somewhat agree, neither agree nor disagree, somewhat disagree, and strongly disagree. The heatmap shows the percentage of respondents selecting each category for statements related to features of chronicity, diagnostic markers, and normal histologic findings in the colon in inflammatory bowel disease. |
Attitudes towards histologic indices/scoring systems in UC
The majority of pathologists (n = 37; 64.9%) were not in favor of including a standardized histologic index in routine pathology reporting for UC. Among the 20 pathologists (35.1%) who supported routine reporting of one of the indices, the NI was the most preferred system (n = 10; 50%), followed by the GS (n = 3; 15%) and the IBD-DCA score (n = 3; 15%). Of those in favor of routine reporting, 11/20 (55%) pathologists preferred that histologic scoring be included in the final diagnostic line, 5/20 (25%) favored inclusion within a synoptic report, while 2/20 (10%) preferred reporting it within a diagnostic or microscopic comment.
Regarding the role of AI in histologic assessment of UC, 27 pathologists (47%) perceived a “probable” or “definite” role for AI in automating histologic scoring for disease activity in UC. In contrast, 25 pathologists (44%) were undecided, while a minority (n = 5; 8%) believed that AI had no role in this setting.
| Discussion | ▴Top |
Our survey included responses from 57 pathologists, mainly from the United States of America and Canada, most of whom were affiliated with academic centers and had fellowship training in gastrointestinal pathology. Nearly two-thirds of the respondents evaluated > 10 biopsies per week from patients with IBD and recognized the clinical relevance of grading histologic activity, particularly in UC. However, an equal number of respondents acknowledged limited familiarity with the established histologic criteria for remission using recognized indices such as the NI, GS, and RHI. This knowledge gap, coupled with a lack of consensus guidelines from gastroenterology and pathology societies in the United States, likely contributes to the slow adoption of standardized histologic scoring systems in routine pathology practice.
Our findings align with prior surveys on this issue but also highlight regional differences, particularly the higher representation of pathologists in the US and Canada. Unlike earlier surveys, which primarily included gastroenterologists with only a small proportion of pathologists, our study focused solely on pathologists. We specifically targeted US-based pathologists because there is a lack of recommendations for how histologic activity in UC should be recorded or which histologic index, if any, should be used. In one global survey of physicians, 312 (87%) of whom were gastroenterologists, two-thirds reported that standard indices were either not used at their centers or were confined to clinical trials and other research settings. Among the 79 physicians (23%) who did use histologic scoring in practice, this was more common in high-volume centers with IBD-dedicated pathologists [6]. Similarly, another survey including 77 gastroenterologists and 12 pathologists from Australia, reported that nearly two-thirds of gastroenterologists who took their survey valued histologic remission over endoscopic remission. While 59% of gastroenterologists and 50% of pathologists in this study expressed a desire to use a histological scoring system, only seven respondents (8%) reported using one in routine practice [7]. These findings highlight a substantial disconnect between the perceived importance of histologic remission and the practical barriers to the implementation of histologic scoring systems in clinical practice. Consistent with these reports, only 10 out of 57 (18%) respondents to our survey indicated that gastroenterologists at their facilities requested a standardized histologic score to be included for UC cases, either at initial diagnosis or during therapeutic response monitoring. Despite these barriers, over one-third of pathologists who completed our survey supported the inclusion of a standardized histologic index, particularly the NI, recognizing its potential value in routine practice. Other standardized histologic indices such as GS and DCA score were used by some pathologists in their practice in this study, suggesting their potential value in routine practice in UC cases. Preferences for reporting varied, with a majority favoring their inclusion in the final diagnostic line to enhance clinical communication. This openness suggests a pathway to increase awareness and utilization of histologic indices, particularly if further integrated into clinical guidelines and supported by gastroenterologists.
The incorporation of these indices into routine practice, however, may be challenging for several reasons. Although our study found no significant differences in knowledge scores across institutional settings or fellowship training, this may partly be reflective of the limited and often conflicting evidence on the prognostic value of histologic remission. While some studies suggest that histologic remission predicts clinical relapses and adverse outcomes, others have reported that in UC, histologic remission does not confer additional benefit over endoscopic remission [15-19]. Another contributing factor may be the existence of multiple histologic scoring systems, several of which, including the Mount Sinai Index and IBD-DCA scores [14] used by some of the participants in this survey, are not fully validated. The relatively limited sample size and the anonymous nature of the survey may have also prevented us from capturing potential differences in institutional practices, as it was not possible to determine whether respondents were from the same or different institutions. In addition, variation in how histologic remission is defined across scoring systems may further delay their integration into clinical practice [13]. Unlike in Europe, where initiatives such as the European Crohn’s and Colitis Organization (ECCO) consensus statements have attempted to standardize histologic reporting, no formal guidelines currently exist in the United States for histologic assessment in IBD [20]. This lack of consensus likely contributes to variability in practice and underscores a broader knowledge gap. Notably, the ECCO recommends using the NI in clinical practice, clinical trials, and observational studies due to its validity and simplicity [20]. Supporting this, studies, including a recent multicenter work from our group [12], have demonstrated substantial intra- and inter-observer reproducibility for active disease using the NI. Our survey results further indicate that among pathologists who expressed interest in including a histologic score in their reports, the majority favored the NI (10 of 20 respondents; 50%) reinforcing its utility in real-world settings [10-12]. However, our findings also uncovered limited familiarity with the histologic remission criteria for the three indices more frequently used in clinical trial and research settings, the NI, RHI, and GS. This limited familiarity may stem from variability in the diagnostic criteria across these indices. The NI grades disease activity on a 0 - 4 scale, focusing on the presence or absence of three key features: chronic inflammatory infiltrate, acute inflammatory infiltrate, and ulceration. Histologic remission as defined by the NI is less than grade 2 or the absence of neutrophils in the lamina propria or epithelium [21, 22]. The RHI evaluates the same three features assigning additional points for severity of features generating a continuous score from 0 to 33. Remission is generally defined as RHI ≤ 3, thereby allowing for mild but unequivocal increase in lamina propria neutrophils [23]. The GS additionally assesses structural changes and eosinophil infiltration, using a hierarchical six-grade system (0 - 5) with subgrades for precise classification. A GS score ≥ 3.1, reflecting crypt abscesses, has been shown to predict clinical relapse [24]. Additional factors contributing to unfamiliarity with histologic remission criteria may be due to inconsistent application in clinical practice, limited exposure due to infrequent use, or the absence of requests from gastroenterologists. Of note, only 17.5% of respondents indicated that they were asked by gastroenterologists to report one of these indices in their routine practice.
Emerging technologies, particularly AI-powered algorithms, offer promising solutions to these challenges. Our survey revealed a gap between interest in using AI to aid histologic evaluation in UC and awareness of existing AI tools among respondents. Notably, 47% of respondents perceived a “probable” or “definite” role for AI in automating histologic scoring, suggesting that AI could serve as a valuable support system to standardize assessments, improve reproducibility, and reduce variability. Several AI algorithms have been developed to analyze UC datasets and assist with histologic grading of biopsies. For example, an AI model by Najdawi et al [25], is capable of reliably distinguishing NI grades. Similarly, Peyrin-Biroulet et al [26], employed a combination of four artificial neural networks to recognize different cell types and NI grades with performance comparable to that of four histopathologists. The recently introduced PICaSSO histologic remission index has shown a stronger correlation with endoscopic activity compared to other histologic indices, including the NI. This index focuses on the presence or absence of neutrophils in the epithelium and lamina propria, exhibiting minimal variability among raters due to its simplicity. This index has also been validated through AI models that appear to accurately and reliably predict histologic activity [27, 28]. Despite these advancements, several challenges must be addressed before AI can be widely integrated into pathology practice. Pathologists across different settings may have limited exposure to or training in these tools, contributing to low awareness. For AI to be practical and efficient, it must be seamlessly incorporated into existing pathology reporting workflows. They will also require rigorous validation across diverse datasets and clinical settings to confirm accuracy, generalizability, and reproducibility. Additionally, their adoption may also be slowed because clear guidelines and regulatory pathways for AI tools in pathology are still in the early stages of development, making careful oversight essential to ensure both reliability and patient safety. Our study has several strengths. The survey targeted pathologists engaged in gastrointestinal pathology practice. It also incorporated the most up-to-date information on histologic assessment for UC. However, we acknowledge some limitations. As with all surveys, our findings may not fully represent actual clinical practice. Moreover, since most respondents were based at academic centers, these results may not entirely reflect the views of pathologists who evaluate UC biopsies in community hospitals and private practice settings. Future studies could address these limitations by expanding recruitment to include more community and private practice pathologists and by pairing survey data with real-world reporting audits. Incorporating targeted questions to better understand barriers to adoption, along with structured comparisons of the different indices, would provide a more comprehensive picture. It would also be valuable to examine how perceptions change over time as AI tools improve and gain broader acceptance.
There is a limitation. Since the total number of respondents are below one-fourth of all pathologists who received invitations, there may be a bias existing in the difference of conditions between respondents and non-respondents.
In conclusion, our survey highlights an ongoing need for a standardized approaches to histologic assessment in UC. Collaborative efforts between pathologists, gastroenterologists, and professional societies are essential to developing consensus guidelines to bridge the gap between emerging research and clinical practice. Furthermore, AI offers a promising solution, with recent studies demonstrating its potential to deliver reliable and reproducible histologic grading while minimizing interobserver variability. These advancements could streamline treat-to-target research protocols, facilitate integration into clinical practice, and ultimately enhance patient outcomes.
| Supplementary Material | ▴Top |
Suppl 1. Knowledge and attitude towards use of histologic indices in ulcerative colitis in routine practice.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Dr. Xiuli Liu is an Associate Editor of Gastroenterology Research. All authors declare no other conflicts of interest.
Informed Consent
Completion of the survey was considered as implied consent for the use of the data in analysis and publication. Participants were informed that their responses may be disseminated in aggregated or anonymized form.
Author Contributions
Study conception, design, and statistical data interpretation: XL; supervision and draft revision: XL and ALB; data compilation, statistical analysis, and original draft preparation: KDS and JL. All authors have reviewed and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available upon reasonable request to the corresponding author.
Abbreviations
AI: artificial intelligence; IBD: inflammatory bowel disease; NI: Nancy Index; UC: ulcerative colitis
| References | ▴Top |
- Lobaton T, Bessissow T, Ruiz-Cerulla A, De Hertogh G, Bisschops R, Guardiola J, Van Assche G, et al. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: A prospective multicenter study. United European Gastroenterol J. 2018;6(5):765-772.
doi pubmed - Yoon H, Jangi S, Dulai PS, Boland BS, Prokop LJ, Jairath V, Feagan BG, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology. 2020;159(4):1262-1275.e1267.
doi pubmed - Shehab M, Al Akram S, Hassan A, Alrashed F, Jairath V, Bessissow T. Histological disease activity as predictor of clinical relapse, hospitalization, and surgery in inflammatory bowel disease: systematic review and meta-analysis. Inflamm Bowel Dis. 2024;30(4):563-572.
doi pubmed - Ma C, Sedano R, Almradi A, Vande Casteele N, Parker CE, Guizzetti L, Schaeffer DF, et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterology. 2021;160(7):2291-2302.
doi pubmed - Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583.
doi pubmed - Nardone OM, Iacucci M, Villanacci V, Peyrin-Biroulet L, Ghosh S, Danese S, Parigi TL. Real-world use of endoscopic and histological indices in ulcerative colitis: Results of a global survey. United European Gastroenterol J. 2023;11(6):514-519.
doi pubmed - Pudipeddi A, Fung C, Christensen B, Bryant RV, Subramaniam K, Chetwood J, Paramsothy S, et al. Knowledge and attitudes towards the use of histological assessments in ulcerative colitis by gastroenterologists vs pathologists. World J Gastroenterol. 2023;29(2):378-389.
doi pubmed - Buchner AM, Farraye FA, Iacucci M. AGA Clinical practice update on endoscopic scoring systems in inflammatory bowel disease: commentary. Clin Gastroenterol Hepatol. 2024;22(11):2188-2196.
doi pubmed - Mosli MH, Parker CE, Nelson SA, Baker KA, MacDonald JK, Zou GY, Feagan BG, et al. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2017;5(5):CD011256.
doi pubmed - Arkteg CB, Wergeland Sorbye S, Buhl Riis L, Dalen SM, Florholmen J, Goll R. Real-life evaluation of histologic scores for Ulcerative Colitis in remission. PLoS One. 2021;16(3):e0248224.
doi pubmed - Le HD, Pflaum T, Labrenz J, Sari S, Bretschneider F, Tran F, Lassen A, et al. Interobserver reliability of the nancy index for ulcerative colitis: an assessment of the practicability and ease of use in a single-centre real-world setting. J Crohns Colitis. 2023;17(3):389-395.
doi pubmed - Shenoy KD, Li J, Allende D, Ballentine SJ, Byrnes K, Deepak P, Dessain AG, et al. Interrater reliability of the nancy histologic index in assessing histologic remission in treated ulcerative colitis biopsies: a multi-institutional experience among gastrointestinal pathologists in the United States. J Clin Transl Pathol. 2025;5(2):54-60.
doi - Vespa E, D'Amico F, Sollai M, Allocca M, Furfaro F, Zilli A, Dal Buono A, et al. Histological scores in patients with inflammatory bowel diseases: the state of the art. J Clin Med. 2022;11(4):939.
doi pubmed - Lang-Schwarz C, Agaimy A, Atreya R, Becker C, Danese S, Flejou JF, Gassler N, et al. Maximizing the diagnostic information from biopsies in chronic inflammatory bowel diseases: recommendations from the Erlangen International Consensus Conference on Inflammatory Bowel Diseases and presentation of the IBD-DCA score as a proposal for a new index for histologic activity assessment in ulcerative colitis and Crohn's disease. Virchows Arch. 2021;478(3):581-594.
doi pubmed - George LA, Feldman HT, Alizadeh M, Abutaleb A, Zullow S, Hine A, Stashek K, et al. Histologic inflammation can predict future clinical relapse in ulcerative colitis patients in endoscopic remission. Crohns Colitis 360. 2023;5(4):otad059.
doi pubmed - Zhao M, Riis LB, Lo B, Attauabi M, Ovesen PD, Wewer MD, Larsen L, et al. Histological, but neither clinical nor endoscopic activity predicts the risk of colectomy in patients with ulcerative colitis treated with biologics. Inflamm Bowel Dis. 2025;31(8):2134-2143.
doi pubmed - Narula N, Aruljothy A, Alshahrani AA, Fadida M, Al-Saedi M, Marshall JK, Rubin DT, et al. Histologic remission does not offer additional benefit for ulcerative colitis patients in endoscopic remission. Aliment Pharmacol Ther. 2020;52(11-12):1676-1682.
doi pubmed - Angyal D, Balogh F, Bessissow T, Wetwittayakhlang P, Ilias A, Gonczi L, Lakatos PL. The role of histology alongside clinical and endoscopic evaluation in the management of IBD-A narrative review. J Clin Med. 2025;14(7):2485.
doi pubmed - Di Vincenzo F, Quintero MA, Serigado JM, Koru-Sengul T, Killian RM, Poveda J, England J, et al. Histologic and endoscopic findings are highly correlated in a prospective cohort of patients with inflammatory bowel diseases. J Crohns Colitis. 2025;19(6):jjae141.
doi pubmed - Magro F, Doherty G, Peyrin-Biroulet L, Svrcek M, Borralho P, Walsh A, Carneiro F, et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis. 2020;14(11):1503-1511.
doi pubmed - Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, Diebold MD, et al. Development and validation of the Nancy histological index for UC. Gut. 2017;66(1):43-49.
doi pubmed - Marchal-Bressenot A, Scherl A, Salleron J, Peyrin-Biroulet L. A practical guide to assess the Nancy histological index for UC. Gut. 2016;65(11):1919-1920.
doi pubmed - Park J, Kang SJ, Yoon H, Park J, Oh HJ, Na HY, Lee HS, et al. Histologic evaluation using the robarts histopathology index in patients with ulcerative colitis in deep remission and the Association of Histologic Remission with Risk of Relapse. Inflamm Bowel Dis. 2022;28(11):1709-1716.
doi pubmed - Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404-409.
doi pubmed - Najdawi F, Sucipto K, Mistry P, Hennek S, Jayson CKB, Lin M, Fahy D, et al. Artificial intelligence enables quantitative assessment of ulcerative colitis histology. Mod Pathol. 2023;36(6):100124.
doi pubmed - Peyrin-Biroulet L, Adsul S, Stancati A, Dehmeshki J, Kubassova O. An artificial intelligence-driven scoring system to measure histological disease activity in ulcerative colitis. United European Gastroenterol J. 2024;12(8):1028-1033.
doi pubmed - Gui X, Bazarova A, Del Amor R, Vieth M, de Hertogh G, Villanacci V, Zardo D, et al. PICaSSO Histologic Remission Index (PHRI) in ulcerative colitis: development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut. 2022;71(5):889-898.
doi pubmed - Iacucci M, Parigi TL, Del Amor R, Meseguer P, Mandelli G, Bozzola A, Bazarova A, et al. Artificial intelligence enabled histological prediction of remission or activity and clinical outcomes in ulcerative colitis. Gastroenterology. 2023;164(7):1180-1188.e1182.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.