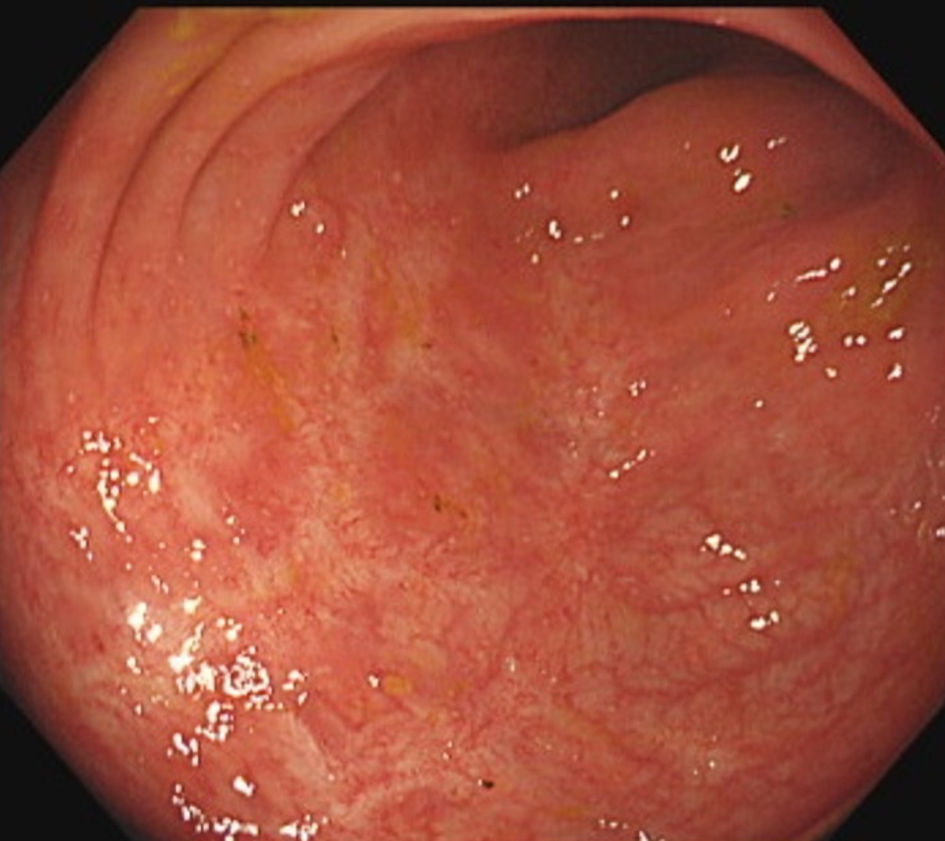
Figure 1. Colonoscopy revealed ulcer scars throughout the large intestine, but the mucosa appeared to be in remission.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Case Report
Volume 17, Number 5-6, December 2024, pages 217-223
Treatment of Crohn’s Disease With Infliximab and Subsequent Development of Takayasu’s Arteritis
Figures

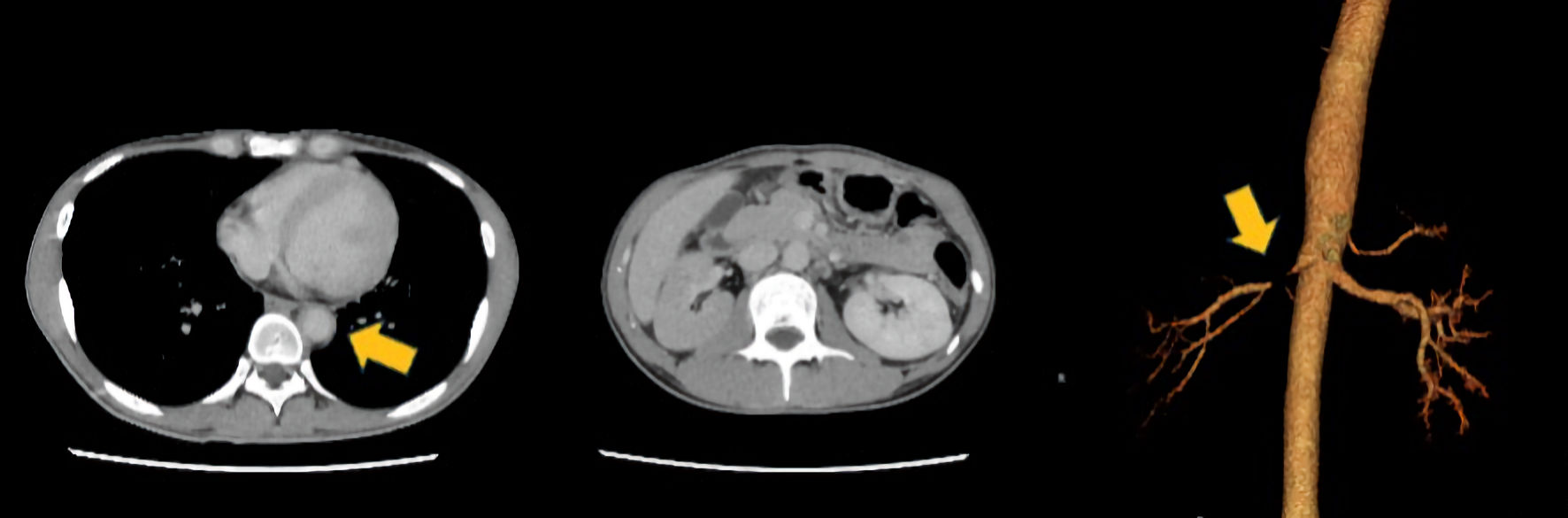
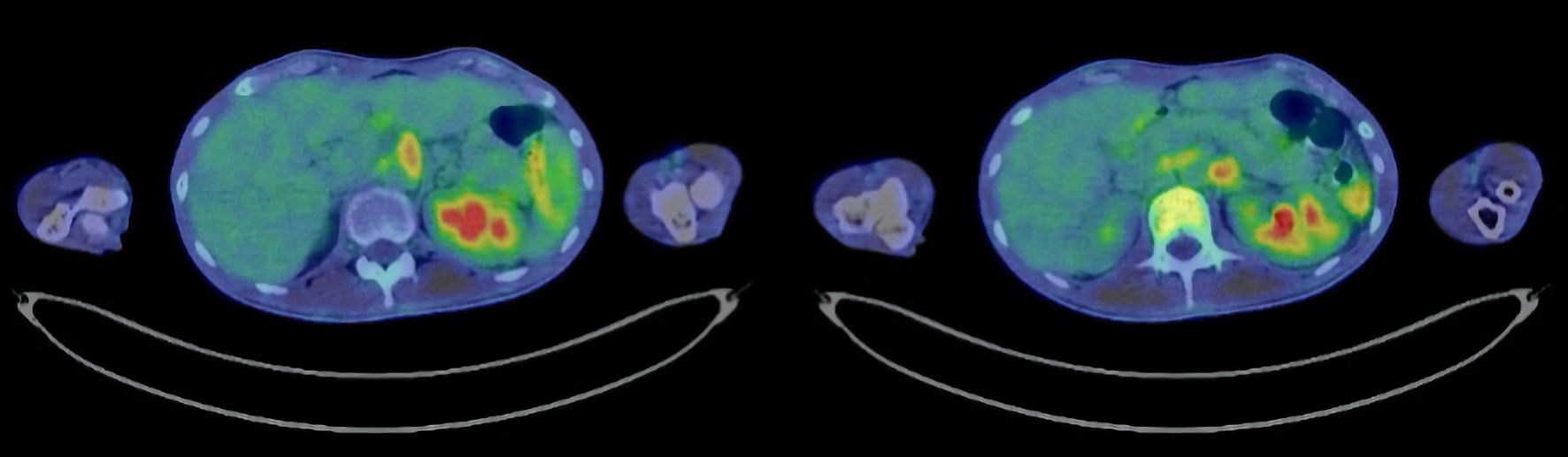
Tables
| ANA: anti-nuclear antibodies; APTT: activated partial thromboplastin time; MPO-ANCA: myeloperoxidase anti-neutrophil cytoplasmic antibodies; PR3-ANCA: proteinase 3 ANCA; PT-INR: prothrombin time-international normalized ratio; sIL-2R: soluble interleukin-2 receptor. | |
| White blood cells | 14,200/µL |
| Red blood cells | 458 × 104/µL |
| Hemoglobin | 13.3 g/dL |
| Hematocrit | 38% |
| Mean corpuscular volume | 83 fL |
| Reticulocytes | 0.9% |
| Platelets | 57.5 × 104/µL |
| PT-INR | 1.36 |
| APTT/con | 55.6/33.6 s |
| Aspartate aminotransferase | 45 U/L |
| Alanine aminotransferase | 30 U/L |
| Lactate dehydrogenase | 216 U/L |
| Alkaline phosphatase | 53 U/L |
| Total protein | 8.4 g/dL |
| Albumin | 3 g/dL |
| Blood urea nitrogen | 11 mg/dL |
| Creatinine | 0.91 mg/dL |
| Uric acid | 3.3 mg/dL) |
| Sodium | 132 mEq/L |
| Potassium | 3.8 mEq/L |
| Chloride | 90 mEq/L |
| Creatinine kinase | 47 U/L |
| Total bilirubin | 0.47 mg/dL |
| Iron | 25 µg/dL |
| Total iron-binding capacity | 158 µg/dL |
| Unsaturated iron-binding capacity | 133 µg/dL |
| Ferritin | 584 ng/dL |
| C-reactive protein | 17.2 mg/dL |
| ANA | < 1:20 |
| PR3-ANCA | 2.4 U/mL |
| MPO-ANCA | < 1.0 U/mL |
| Carcinoembryonic antigen | 3.6 ng/mL |
| Carbohydrate antigen 19-9 | 12 U/mL |
| sIL-2R | 1,390 U/mL |
| References | Age | Sex | Diagnostic order | Type of CD | Anti-TNF-α agent | Clinical presentation | Steroid for TA | Tests to diagnose TA |
|---|---|---|---|---|---|---|---|---|
| F: female; M: male; ADA: adalimumab; CD: Crohn’s disease; CT: computed tomography; CTA: computed tomography angiography; IFX: infliximab; MRI: magnetic resonance imaging; ND: not determined; NMR: nuclear magnetic resonance; PET: positron emission tomography; TA: Takayasu’s arteritis; TNF: tumor necrosis factor; US: ultrasonography. | ||||||||
| Domenech et al, 2005 [5] | 27 | F | CD → TA | Ileocolonic | IFX | Fever, epigastric pain, vomiting | 1 mg/kg/day | CT, angio-NMR |
| Kellermayer et al, 2008 [6] | 17 | F | CD → TA | Colonic | IFX | Nausea, emesis | None | Contrast-enhanced CT, MRI |
| El-Matary et al, 2009 [7] | 15 | F | CD → TA | Ileocolonic | IFX | Fever, headache, vomiting, abdominal pain | Done | CT, MRI angiogram |
| Katoh et al, 2010 [8] | 20 | F | CD → TA | Ileocolonic | IFX | Anterior neck pain | 40 mg/day | Contrast-enhanced CT, US |
| Okada et al, 2010 [9] | 30 | M | CD → TA | Ileocolonic | IFX | Neck pain | 60 mg/day | Contrast-enhanced CT, MRI, CTA, PET |
| Osman et al, 2011 [10] | 17 | F | CD → TA | ND | IFX | ND | 20 mg/day | Contrast-enhanced CT |
| Kiyohara et al, 2015 [11] | 23 | F | CD → TA | Ileocolonic | ADA | Fever, abdominal pain | 40 mg/day | Contrast-enhanced CT |
| Sy et al, 2016 [12] | 13 | M | CD → TA | ND | IFX | ND | Done | ND |
| 15 | F | CD → TA | ND | IFX | ND | None | ND | |
| 8 | F | CD → TA | ND | IFX | ND | Done | ND | |
| 9 | F | CD → TA | ND | IFX | ND | Done | ND | |
| Miyakawa et al, 2016 [13] | 19 | M | CD → TA | Ileocolonic | IFX | Fever | 40 mg/day | Contrast-enhanced CT, CTA |
| Takeuchi et al, 2020 [14] | 10 | M | CD → TA | Ileocolonic | IFX, ADA | Neck pain | 30 mg /day | Contrast-enhanced CT |
| Kollen et al, 2020 [15] | 6 | M | CD → TA | Ileocolonic | IFX | Back and chest pain, fever | Done | CT, MRI, PET |
| Kishimoto et al, 2021 [16] | 14 | M | CD → TA | Ileocolonic | IFX | Fever, pain in right arm | 15 mg/day | Contrast-enhanced CT, PET |
| Fotis et al, 2022 [17] | 15 | F | CD → TA | Ileocolonic | IFX | Fever | 40 mg /day | CT, MRI angiogram, PET |
| Ariga et al, 2024 [18] | 23 | F | CD → TA | Colonic | IFX | Abdominal pain | Done | CT, PET |
| Present patient | 23 | M | CD → TA | Colonic | IFX | Fever, headache | 50 mg/day | Contrast enhanced CT, CTA, PET |