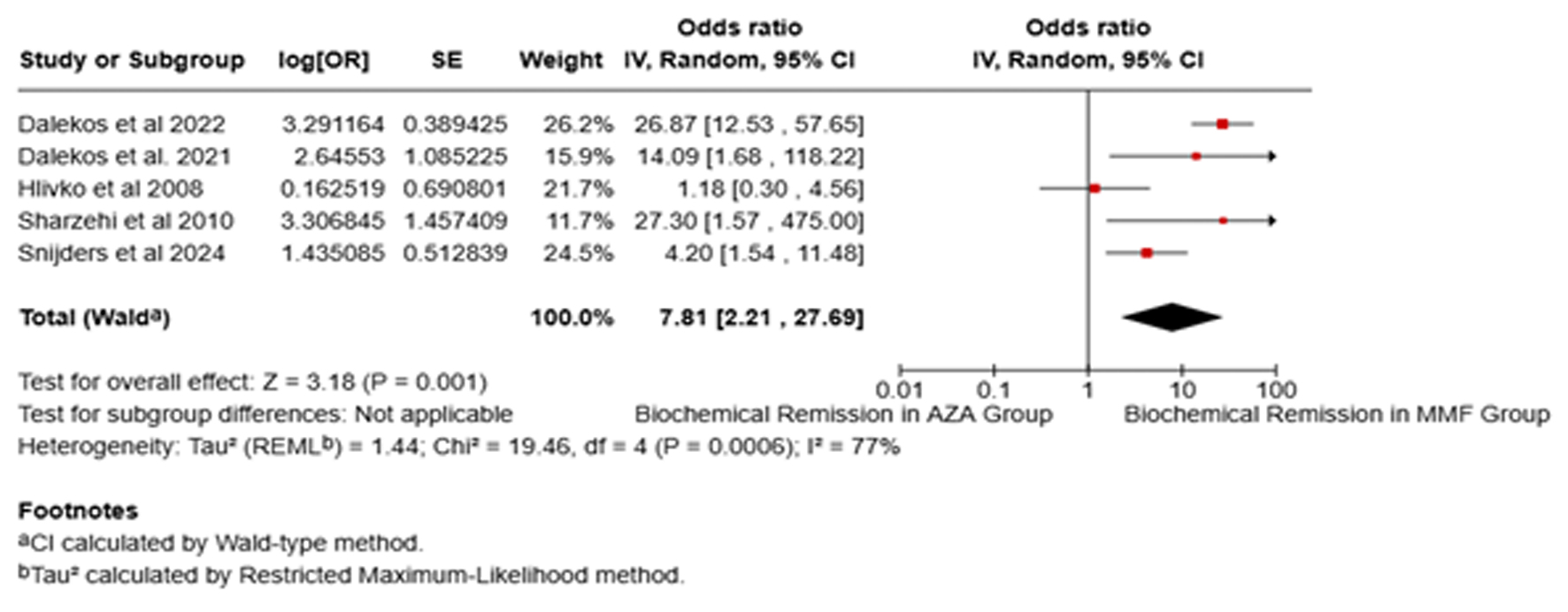
Figure 1. Forest plot of comparison of mycophenolate mofetil (MMF) with azathioprine (AZA) for biochemical remission.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 4, August 2025, pages 164-174
Efficacy and Safety of Mycophenolate Mofetil Compared to Azathioprine in Autoimmune Hepatitis: A Meta-Analysis
Figures


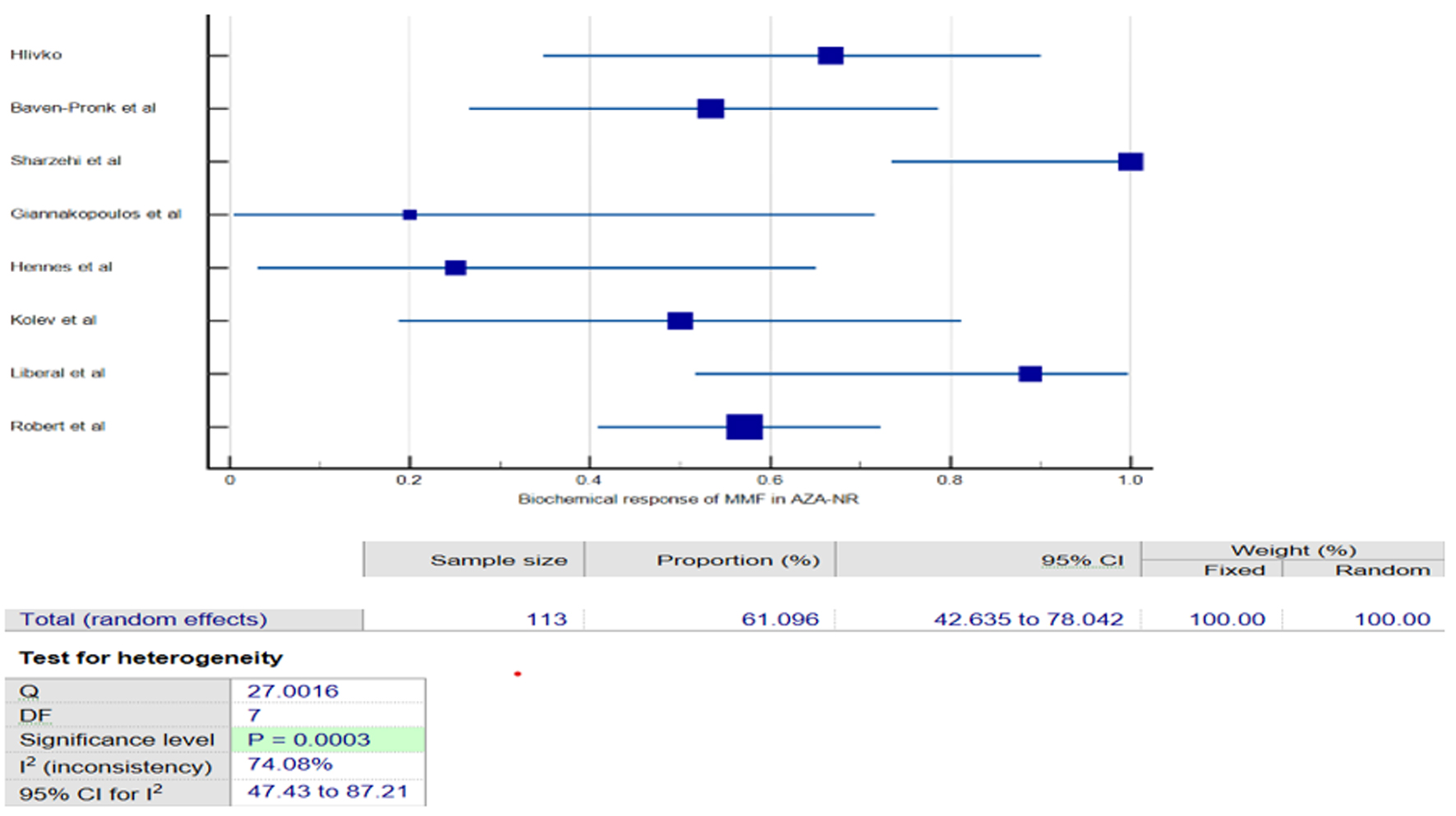
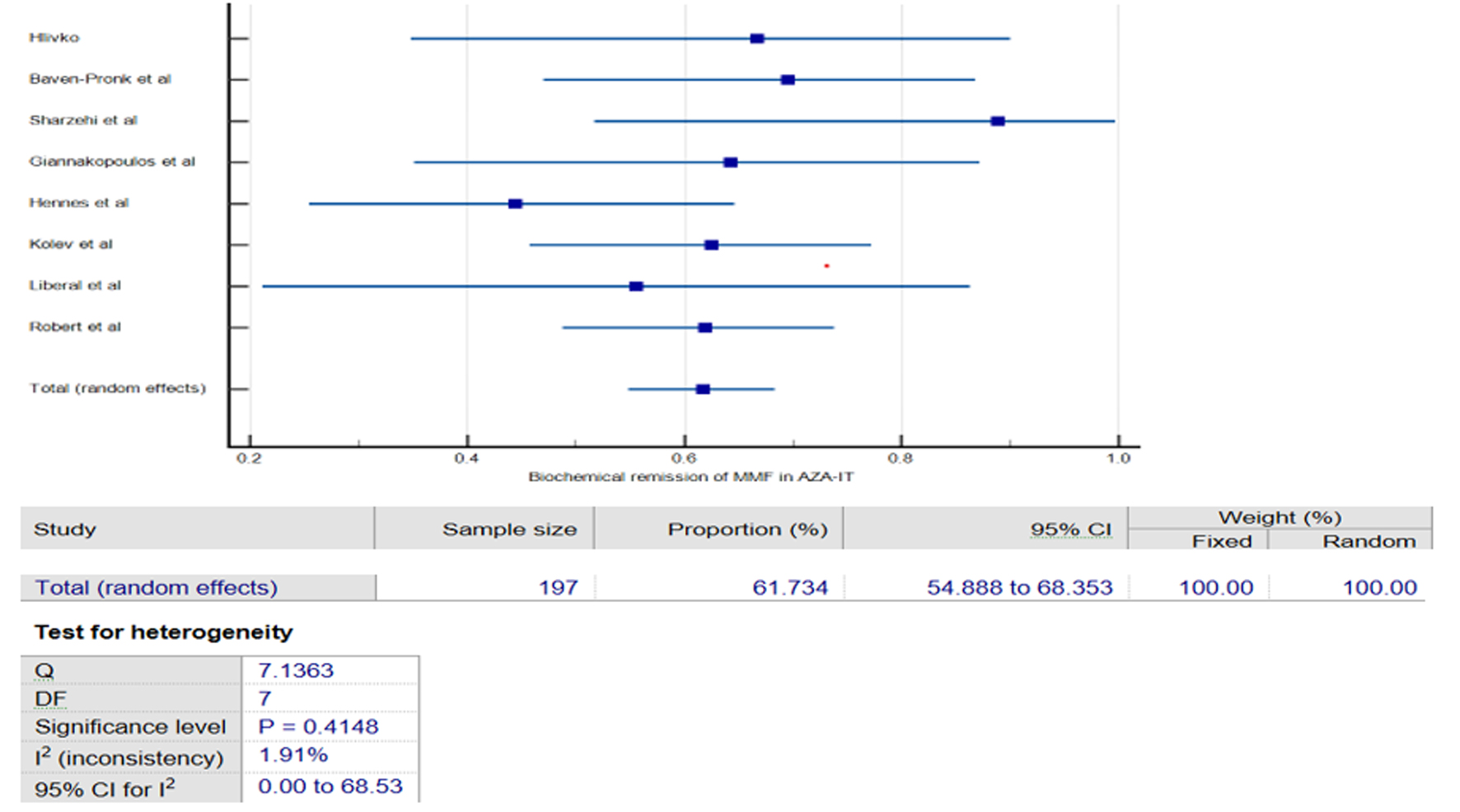
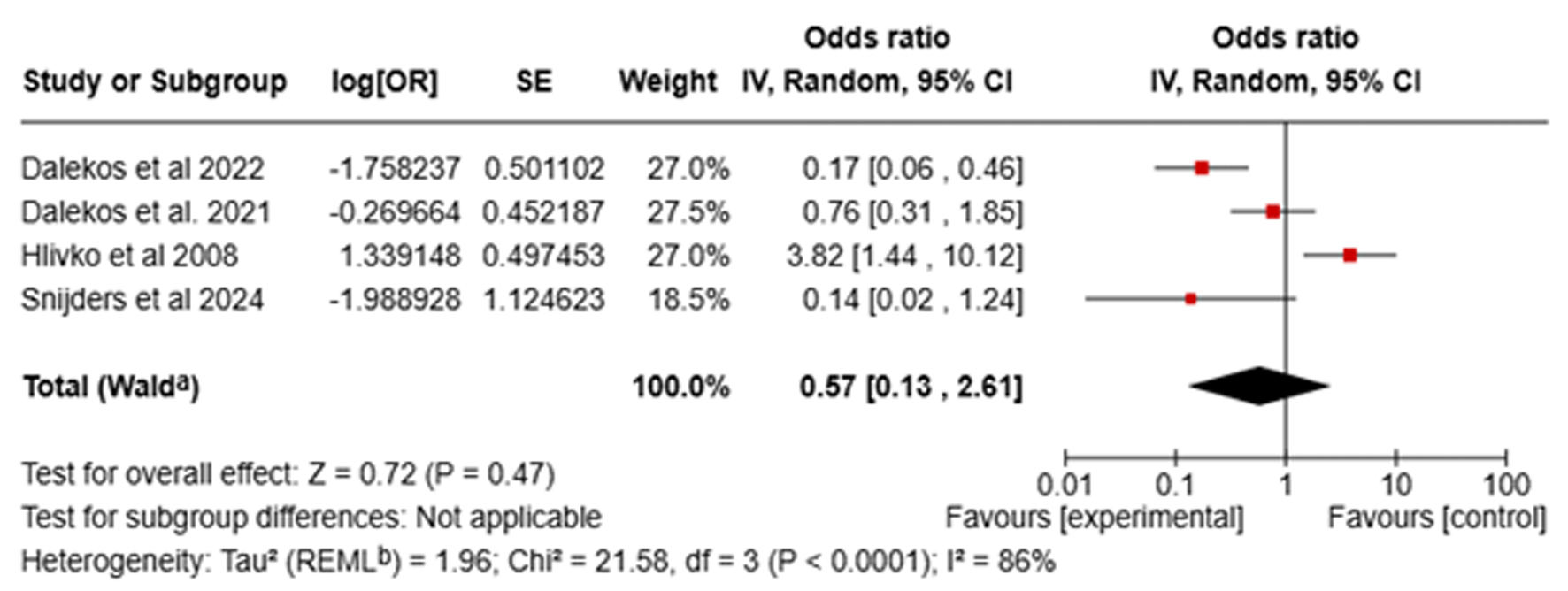
Tables
| Study | Design | Country | Year of study | Study groups | Definition of autoimmune hepatitis | Follow-up duration (months) | Patients received MMF | Patients received AZA | Cirrhosis, N (%) | Age (mean), treatment/control | Sex (female) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AZA: azathioprine; AZA-IT: azathioprine intolerant; AZA-NR: azathioprine non-respondent; IAHG: International Autoimmune Hepatitis Group; MMF: mycophenolate mofetil. | |||||||||||
| Hlivko et al [13] | Retrospective | USA | 2008 | AZA vs. MMF | AASLD criteria | 13 | 17 | 92 | 22% | 42.8 ± 16.9 | 82.8% |
| Giannkapoulos et al [14] | Retrospective multicenter | Sweden | 2018 | AZA vs. MMF | AASLD criteria | 0-24 | 22 | 22 | 6 | 46.5/50.5 | 54.5% |
| Baven-Pronk et al [15] | Retrospective multicentric | Netherlands/Belgium | 2011 | AZA-IT/AZA-NR | IAHG criteria | 39 | 15 | 15 | 11 (37.7) | 38/35 | 86.7% |
| Sharzehi et al [16] | Retrospective | USA | 2010 | AZA-IT/AZA-NR | Clinical/serologic/biochemical/histologic | 42 | 21 | 20 | 46.3/55.7 | 75% | |
| Roberts et al [17] | Retrospective | Australia | 2018 | AZA-IT/AZA-NR | IAIHG | 34 | 105 | 94 | 38 (37) | 50/52 | 83.3% |
| Liberal et al [18] | Retrospective | Portugal | 2021 | AZA-IT/AZA-NR | IAIHG | 78 | 18 | 18 | 4 | 44.5/33.3 | 83% |
| Kolev et al [19] | Retrospective | Switzerland | 2022 | AZA-IT/AZA-NR | IAIHG | 51.5 | 50 | 50 | 4 (8) | 49.5/51 | 82.5% |
| Hennes et al [20] | Retrospective multicenter | Germany and UK | 2008 | AZA-IT/AZA-NR | IAIHG | 24 | 27 | 27 | - | 46/42 | 75% |
| Dalekos et al [21] | Prospective cohort | Greece | 2021 | AZA vs. MMF | IAIHG | 39 | 32 | 32 | MMF: 6 AZA: 6 | 54/55 | 71.8% |
| Dalekos et al [22] | Prospective cohort | Greece | 2022 | AZA vs. MMF | IAIHG | 39 | 183 | 64 | MMF: 38 (20.7) AZA: 12 (18.8) | 49/48 | 73.2% (MMF) 73.4% (AZA) |
| Snijders et al (CAMARO trial) [23] | RCT | Netherland/Belgium | 2024 | AZA vs. MMF | IAIHG | 6 | 39 | 31 | MMF: 10 (26) AZA: 7 (23) | 60 ± 14 56 ± 14.4 | 76.9% (MMF) 70% (AZA) |
| Adverse events | AZA (n = 272), n (%) | MMF (n = 381), n (%) |
|---|---|---|
| AZA: azathioprine; MMF: mycophenolate mofetil. | ||
| Gastrointestinal symptoms | 37 (13.6) | 45 (11.8) |
| Skin abnormalities | 4 (1.5) | 10 (2.6) |
| Hair loss | 6 (1.5) | 4 (1) |
| Myalgias | 4 (1.4) | 5 (1.3) |
| Myelotoxicity | 6 (2.2) | 1 (0.3) |
| Hepatotoxicity | 5 (1.8) | 0 |
| Infections | 10 (3.7) | 13 (3.4) |
| Malignancy | 0 | 2 (0.5) |
| Pancreatitis | 1 (0.3) | 0 |