| Median age of diagnosis | 65 years (range 49 - 88) |
| Race | |
| White | 113 (80.14%) |
| African American | 22 (15.60%) |
| Other | 6 (4.26%) |
| Sex | |
| Male | 113 (80.14%) |
| Female | 28 (19.86%) |
| History of hepatitis B infection | 8 (5.67%) |
| History of hepatitis C infection | 72 (51.06%) |
| History of alcoholic liver cirrhosis | 110 (78.01%) |
| History of MAFLD | 17 (12.06%) |
| History of DM | 50 (35.46%) |
| Ascites at diagnosis | 35 (24.82%) |
| HE at diagnosis | 41 (29.08%) |
| Smoking history | |
| Active | 52 (37%) |
| Former | 53 (38%) |
| Never | 36 (25%) |
| Alcohol use | |
| Active | 52 (37%) |
| Never | 65 (46%) |
| Former | 24 (17%) |
| Number of TARE procedures | |
| 1 | 122 (87%)a |
| 2 | 17 (12%) |
| 3 | 2 (1%) |
| Therapy before TARE | |
| None | 50 (35.46%) |
| TACE | 59 (41.84%) |
| Ablation | 35 (24.82%) |
| Surgery | 18 (12.77%) |
| SBRT | 7 (4.97%) |
| Systemic therapy | 12 (8.52%) |
| Number of HCC lesions | |
| 1 | 65 (46.10%) |
| 2 | 30 (21.28%) |
| 3 | 16 (11.35%) |
| > 3 | 30 (21.28%) |
| Size of HCC lesions | |
| ≤ 3 cm | 56 (40%) |
| > 3 cm | 79 (56%) |
| Not measurable | 6 (4%) |
| HCC in liver lobes | |
| Right lobe | 66 (46.81%) |
| Both lobes | 47 (33.33%) |
| Left lobe | 23 (16.31%) |
| Undefined | 5 (3.55%) |
| PVTT | |
| None | 107 (75.89%) |
| Main portal vein | 14 (9.93%) |
| Right portal vein | 9 (6.38%) |
| Present but location is unclear | 7 (4.97%) |
| Left portal vein | 4 (2.84%) |
| Child-Pugh score | |
| A | 105 (74.47%) |
| B | 34 (24.11%) |
| C | 2 (1.42%) |
| Baselineb T bili (mg/dL; median/mean) | 1/1.05 |
| Baselineb albumin (g/dL; median/mean) | 4/3.76 |
| Baselineb INR (median/mean) | 1/1.09 |
| Baselineb AFP (ng/dL; median/mean) | 26.5 (1 - 78,314)/1,688.10 |
| TKI use | |
| No | 107 (76%) |
| Post-TARE | 29 (21%) |
| Pre-TARE | 5 (3%) |
| TKI drugs used | |
| Sorafenib | 21 (14.89%) |
| Lenvatinib | 16 (11.35%) |
| Cabozantinib | 2 (1.42%) |
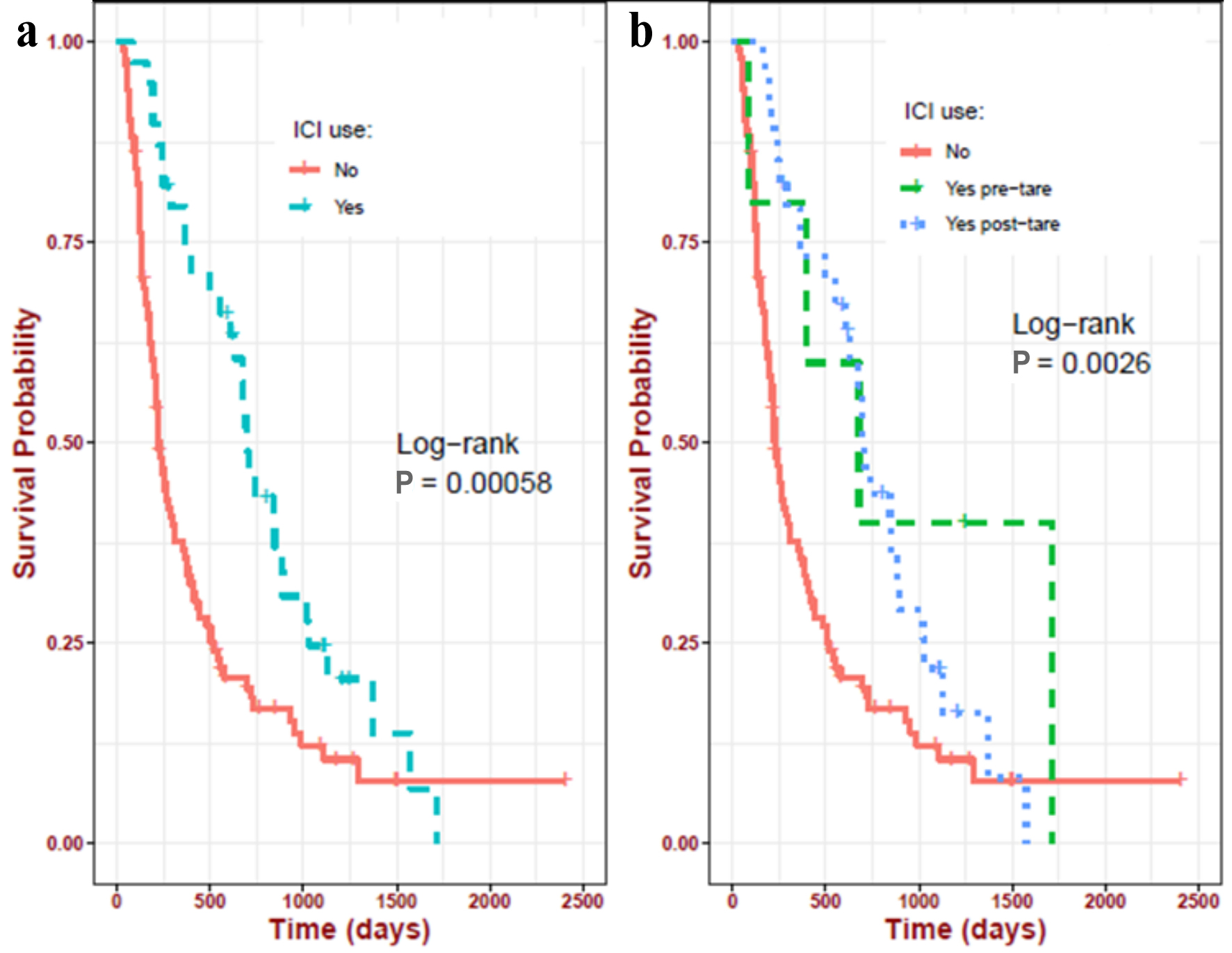
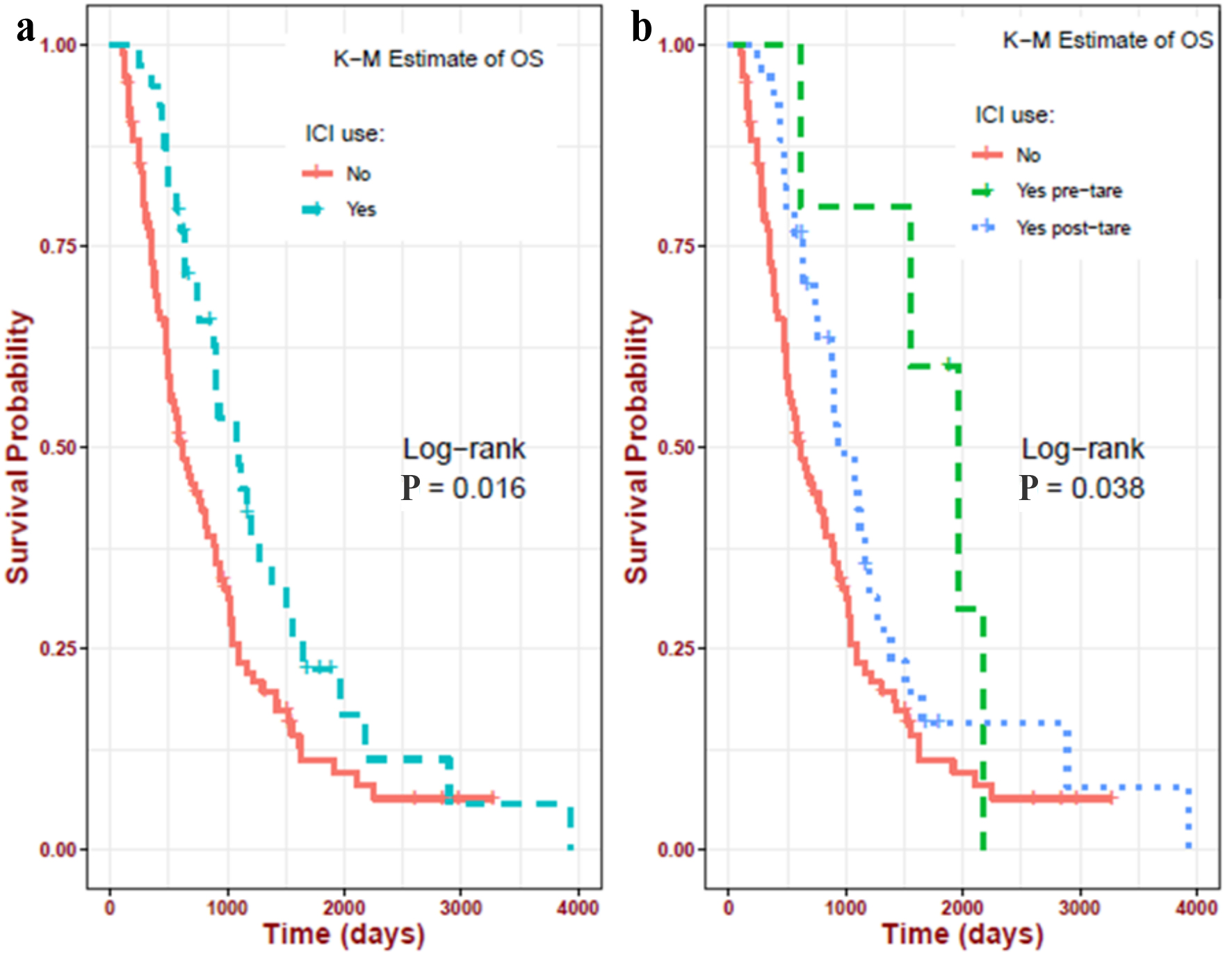
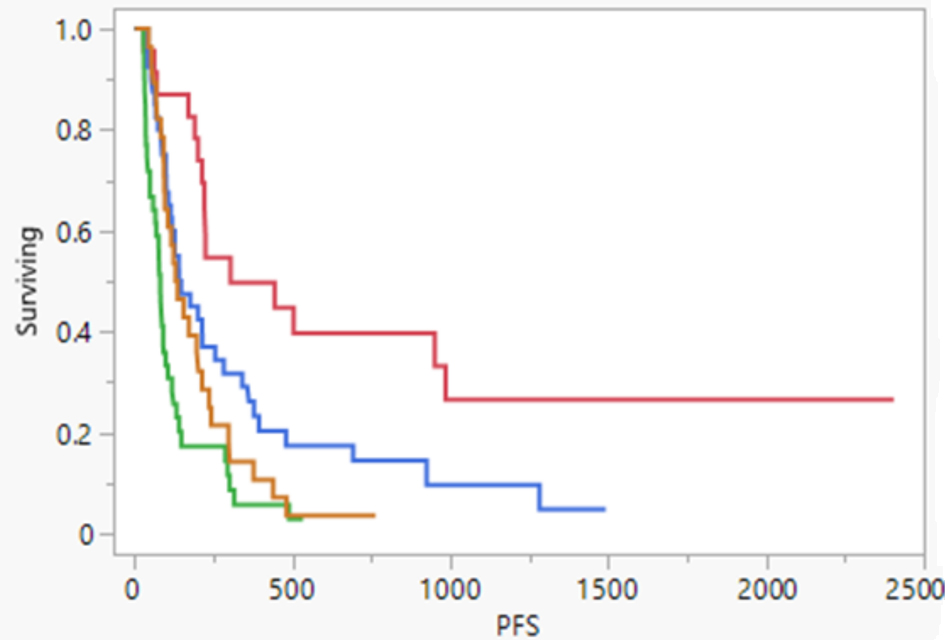
| ICI use | |
| No-ICI group | 102 (72.34%) |
| Post-TARE ICI group | 34 (24.11%) |
| Pre-TARE ICI group | 5 (3.55%) |
| ICI drugs used | |
| Nivolumab | 19 (49%) |
| Atezolizumab and bevacizumab | 20 (51%) |