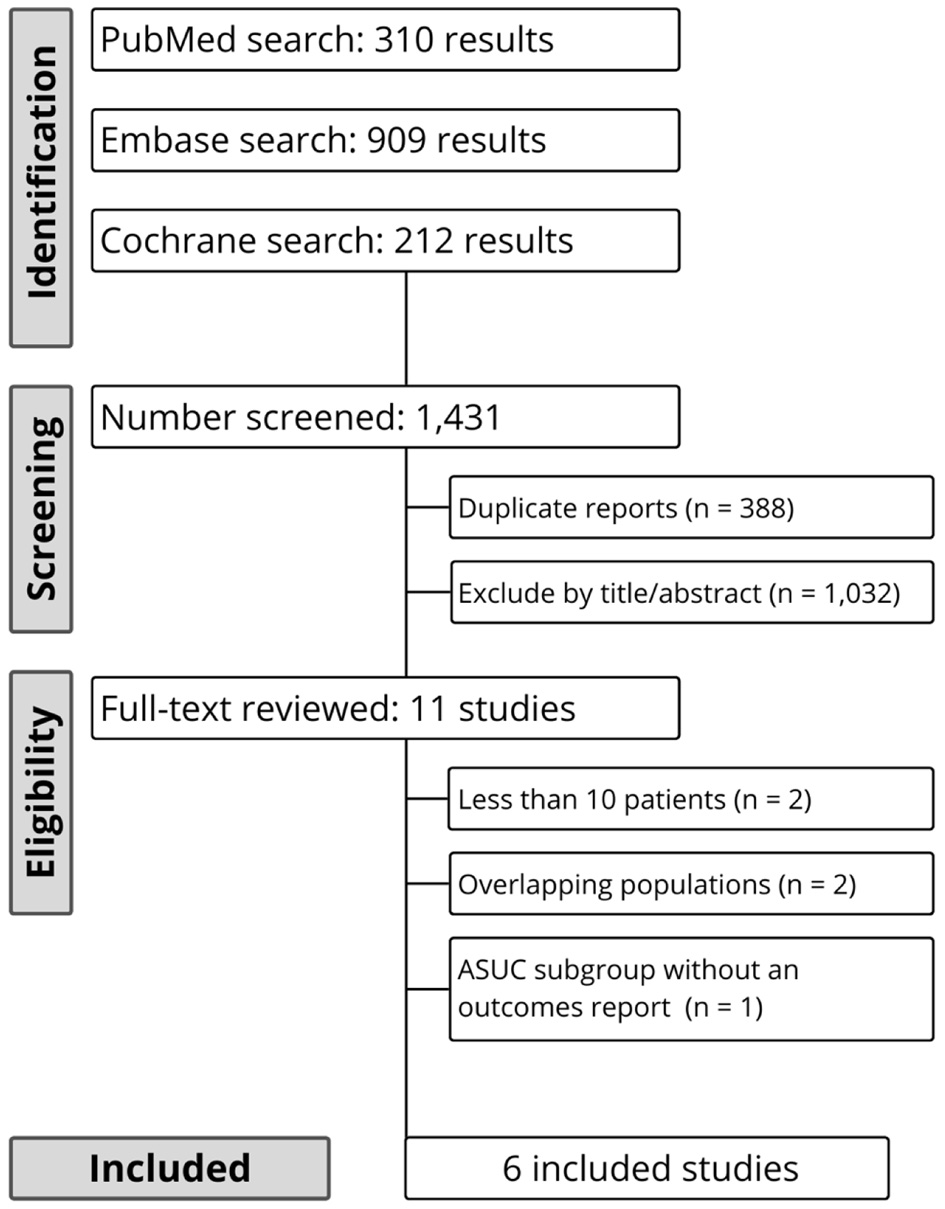
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study screening and selection.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 6, December 2025, pages 299-307
Efficacy and Safety of Tofacitinib for Acute Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis
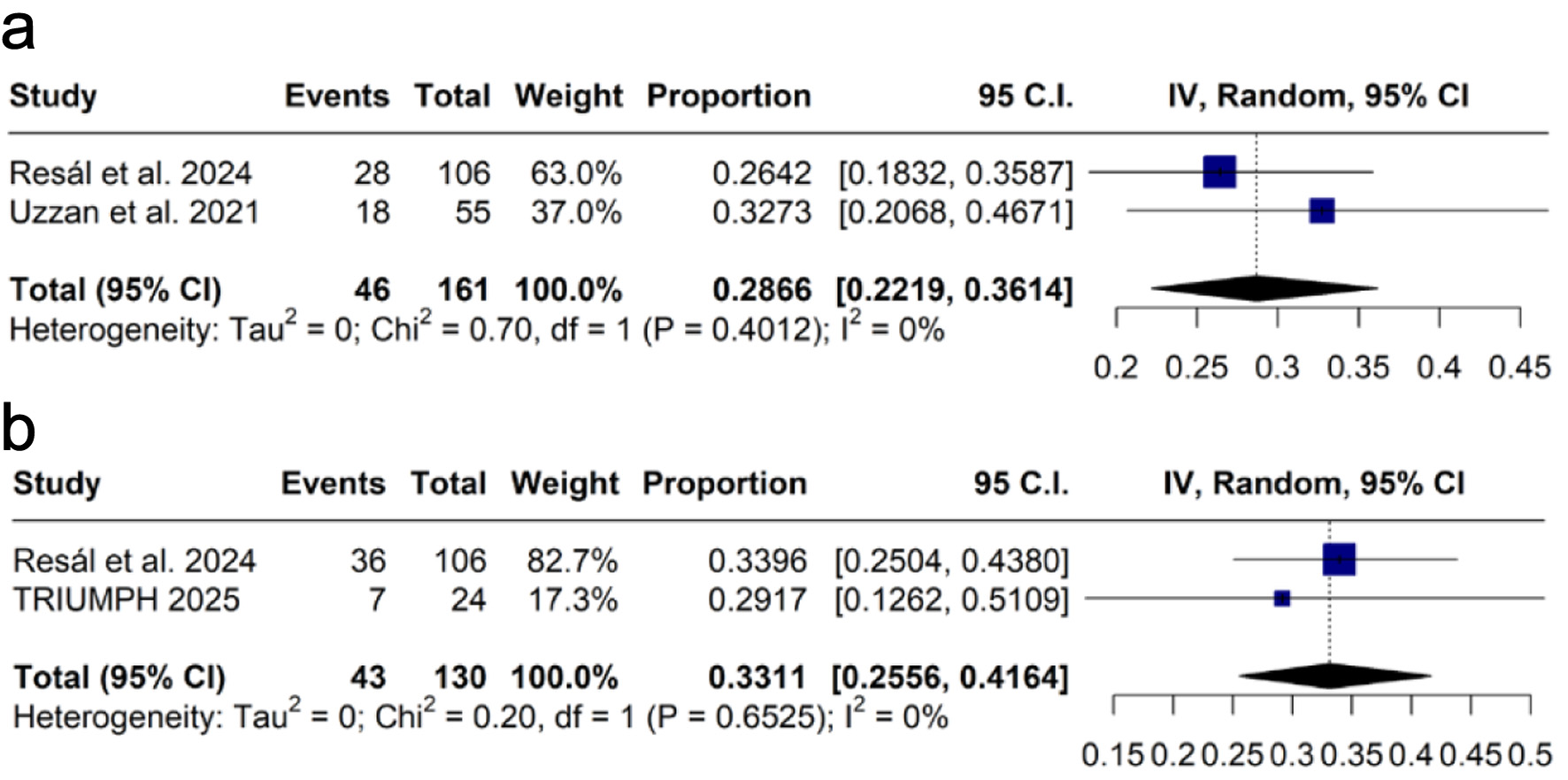
Figures

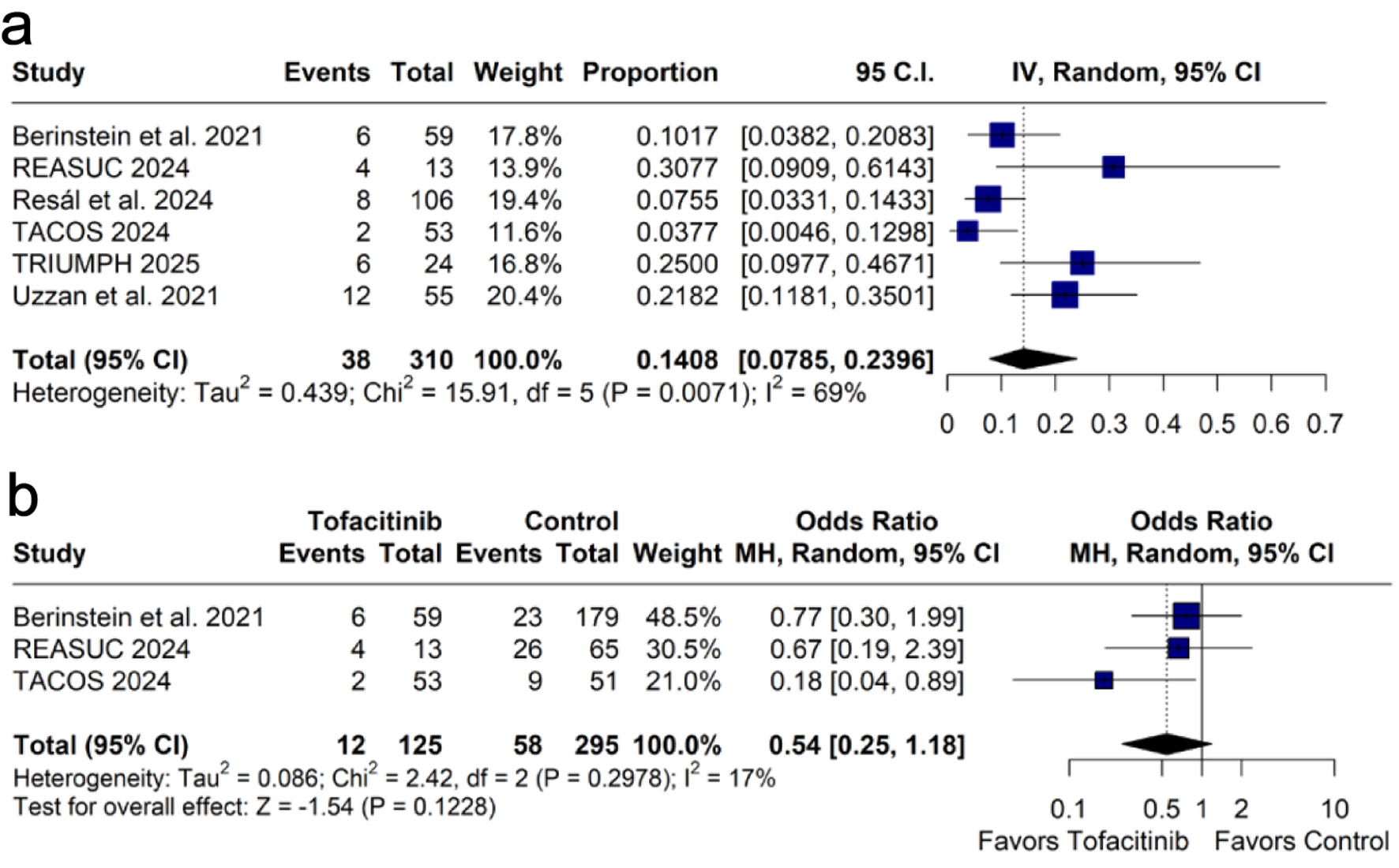
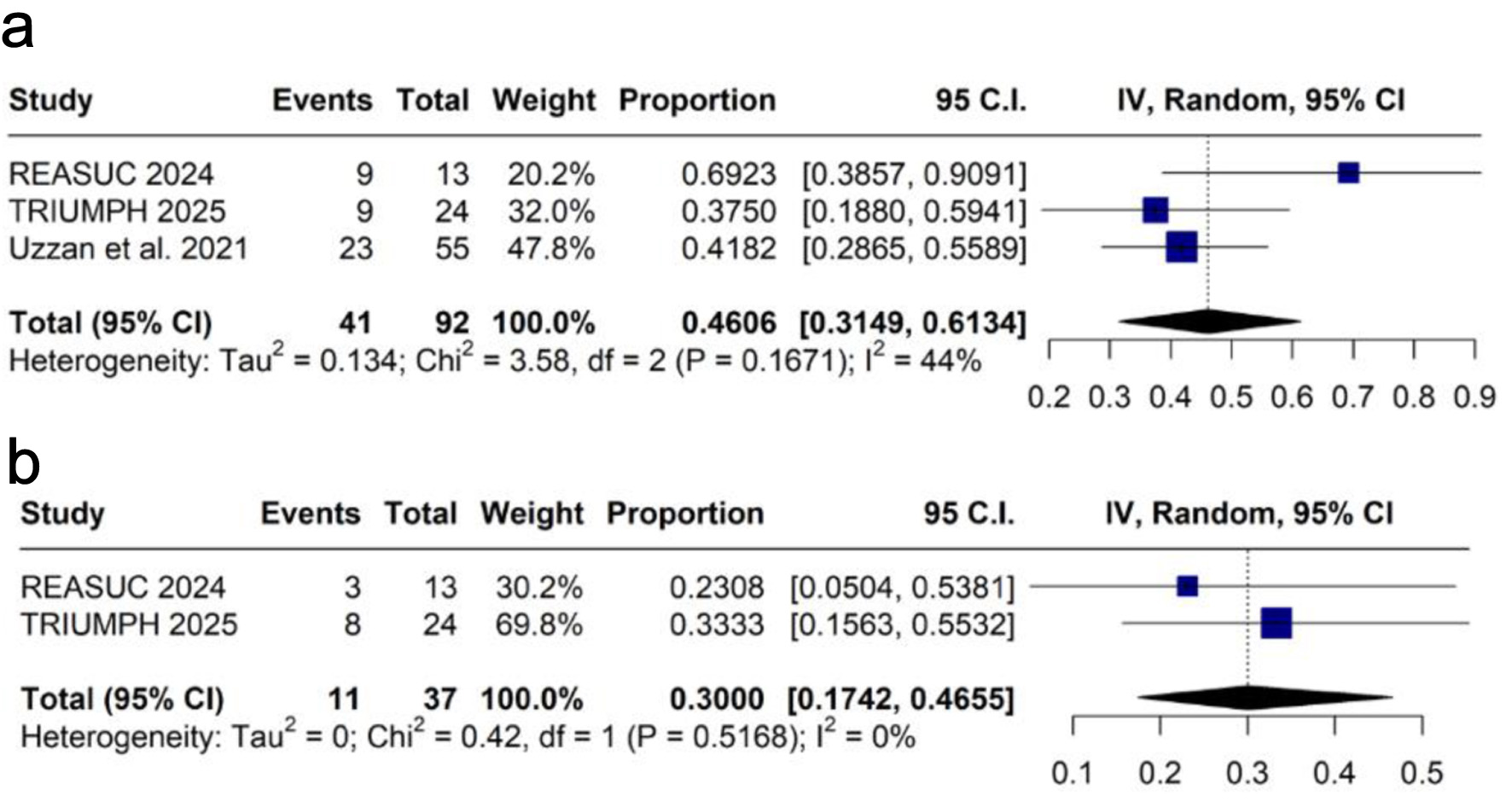
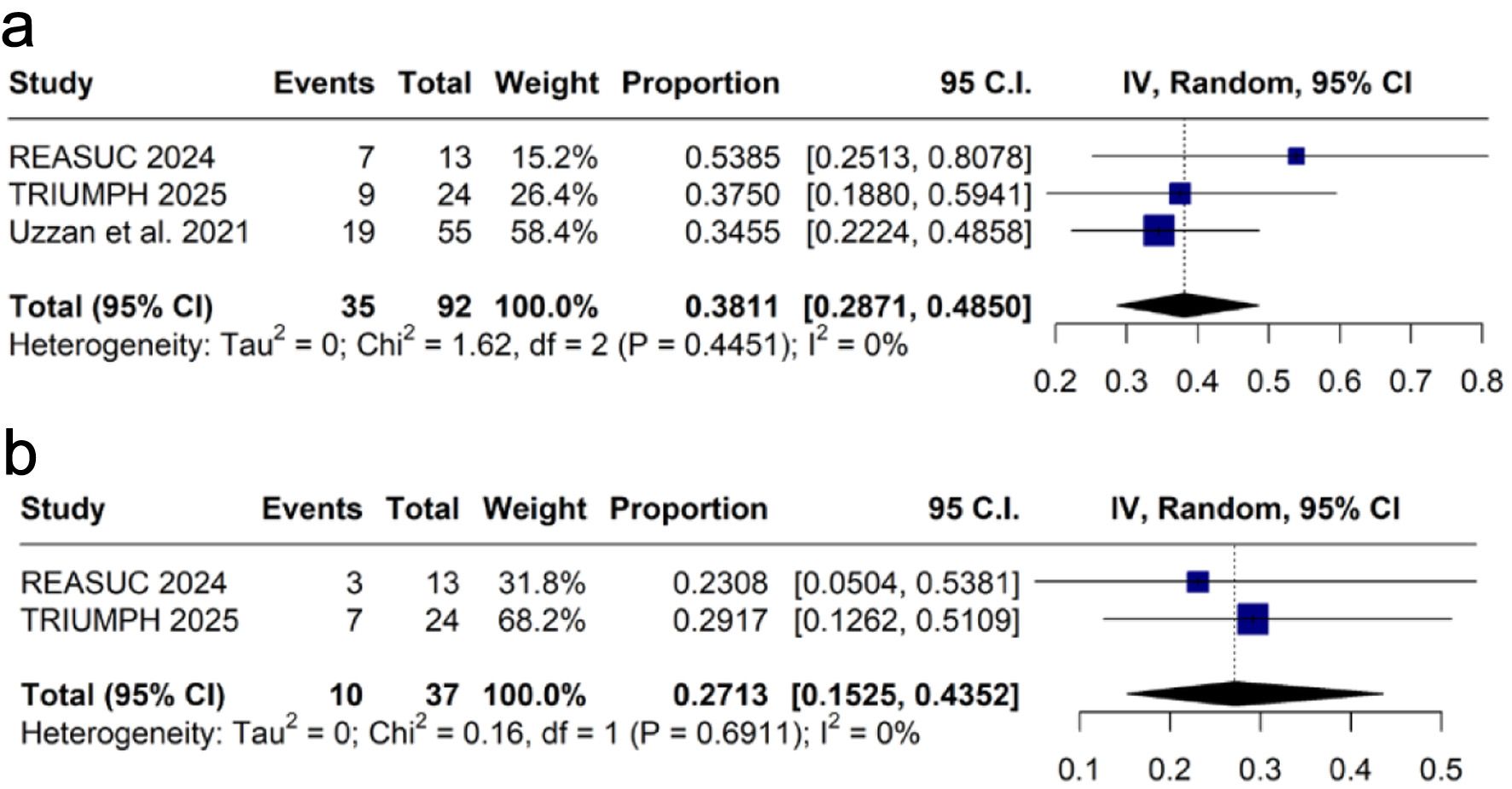
Tables
| Trial | Study design | Country | Patients, n | Mean age, years | Male sex, n (%) | Disease extent | Criteria for ASUC | Mayo Endo Score | Follow-up, weeks |
|---|---|---|---|---|---|---|---|---|---|
| ASUC: acute severe ulcerative colitis; BID: twice daily; IBD: inflammatory bowel disease; Mayo Endo Score: Mayo endoscopic subscore; NA: not available; RCT: randomized controlled trial; TID: three times daily; TNF: tumor necrosis factor. | |||||||||
| TACOS, 2024 [11] | RCT | India | 53 | 37 | 29 (54%) | Proctitis: 4 (7%) Left-sided colitis: 35 (66%) Pancolitis: 14 (26%) | Truelove-Witts criteria | NA | 12 |
| REASUC, 2024 [17] | Retrospective cohort Multicenter | Europe | 13 | NA | NA | NA | Partial Mayo Score or Lichtiger score | NA | 52 |
| Berinstein et al, 2021 [16] | Retrospective case-control | USA | 40 | 34 | 16 (40%) | Proctitis: 1 (2.5%) Left-sided colitis: 8 (20%) Pancolitis: 31 (77.5%) | Truelove-Witts criteria or severe labs and/or endoscopic disease | Mayo 2: 10 (25%) Mayo 3: 28 (71%) | 12 |
| Uzzan et al, 2021 [19] | Retrospective and prospective cohort Multicenter | France | 55 | 27.6 | 30 (55.5%) | Left-sided colitis: 19 (35%) Pancolitis: 36 (65%) | Hospitalization for a flare-up of the IBD | Mayo 2: 18 (32%) Mayo 3: 37 (67%) | 52 |
| Resal et al, 2024 [18] | Retrospective cohort Multicenter | Europe, Canada, Israel | 106 | 38 | 45 (42%) | Proctitis: 3 (3%) Left-sided colitis: 34 (32%) Pancolitis: 69 (65%) | Truelove-Witts criteria | Median score of 3 | 52 |
| TRIUMPH, 2025 [12] | Prospective open-label trial Multicenter | Canada | 24 | 42.5 | 13 (54%) | Left-sided colitis: 2 (8.3%) Extensive colitis: 4 (16.7%) Pancolitis: 18 (75%) | Modified Truelove-Witts criteria | Mayo 3: 18 (75%) | 52 |
| Trial | Group | Tofacitinib dosage (or control) | Concomitant therapy | Prior anti-TNF exposure, % |
|---|---|---|---|---|
| BID: twice daily; NA: not available; N/A: not applicable; TID: three times daily; TNF: tumor necrosis factor. | ||||
| TACOS, 2024 [11] | Intervention | 10 mg TID × 7 days, then 10 mg BID | All received intravenous hydrocortisone (max 7 days), followed by a prednisolone taper | 5.6% |
| Control | Placebo | Same as intervention group | 3.9% | |
| REASUC, 2024 [17] | Intervention | 10 mg BID | All had previously failed treatment with methylprednisolone | N/A |
| Control | Infliximab, cyclosporin | Same as the intervention group | N/A | |
| Berinstein et al, 2021 [16] | Intervention | 10 mg BID (40%) OR 10 mg TID (60%) for 3 days, then 10 mg BID | All methylprednisolone 15 mg every 6 h | 100% |
| Control | Infliximab, cyclosporin | Same as intervention group | 38.9% | |
| Uzzan et al, 2021 [19] | Intervention | 5 mg BID (22%) or 10 mg BID (78%) | 36 (65%) receiving steroids | 89% |
| Control | N/A | N/A | N/A | |
| Resal et al, 2024 [18] | Intervention | 20 mg/d induction, 10 mg/d maintenance | 52 (49%) Prednisolone/methylprednisolone | 83.6% |
| Control | N/A | N/A | N/A | |
| TRIUMPH, 2025 [12] | Intervention | 10 mg BID for 8 weeks, then maintenance 5 or 10 mg BID | All received intravenous steroids for 7 days, followed by taper in responders | 33% |
| Control | N/A | N/A | N/A | |