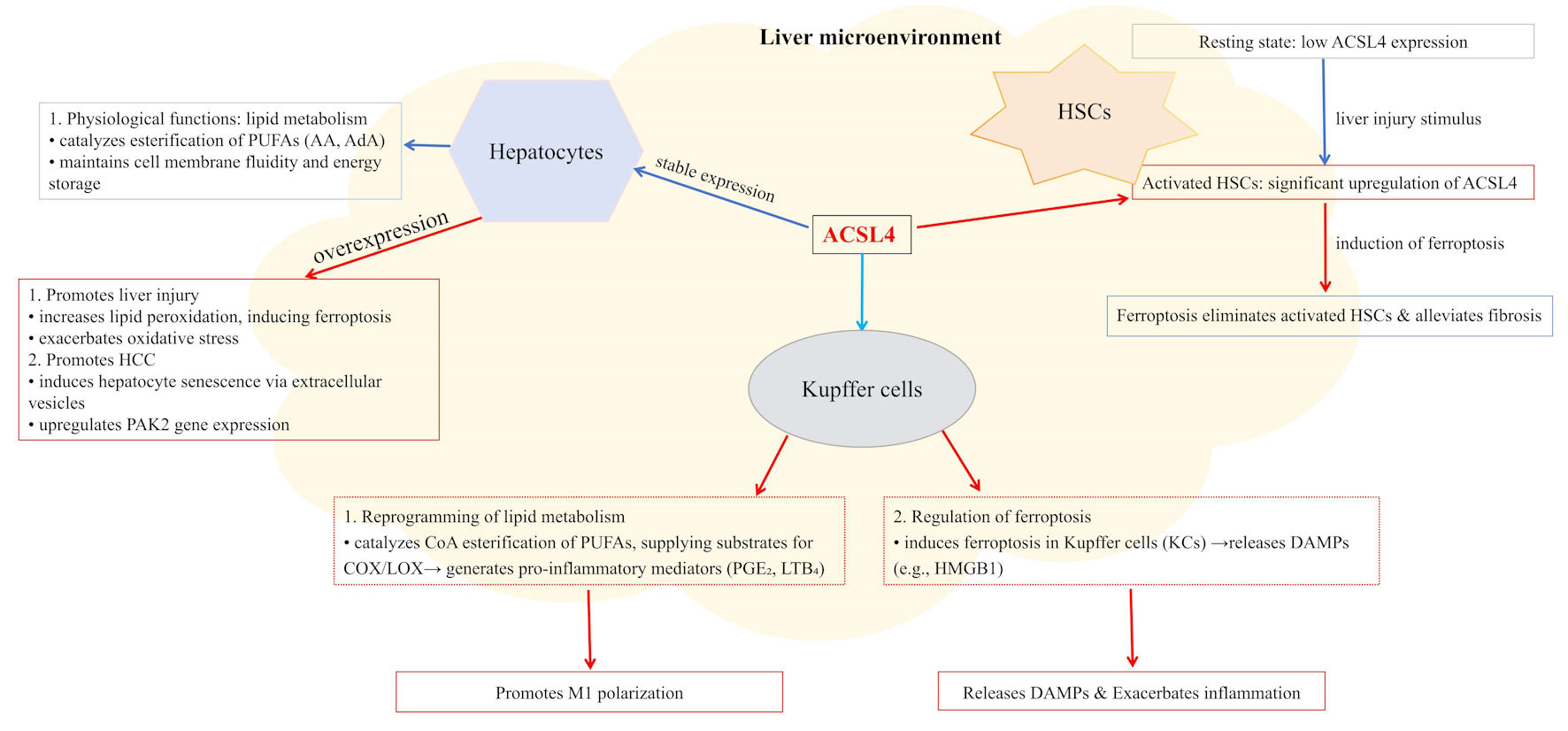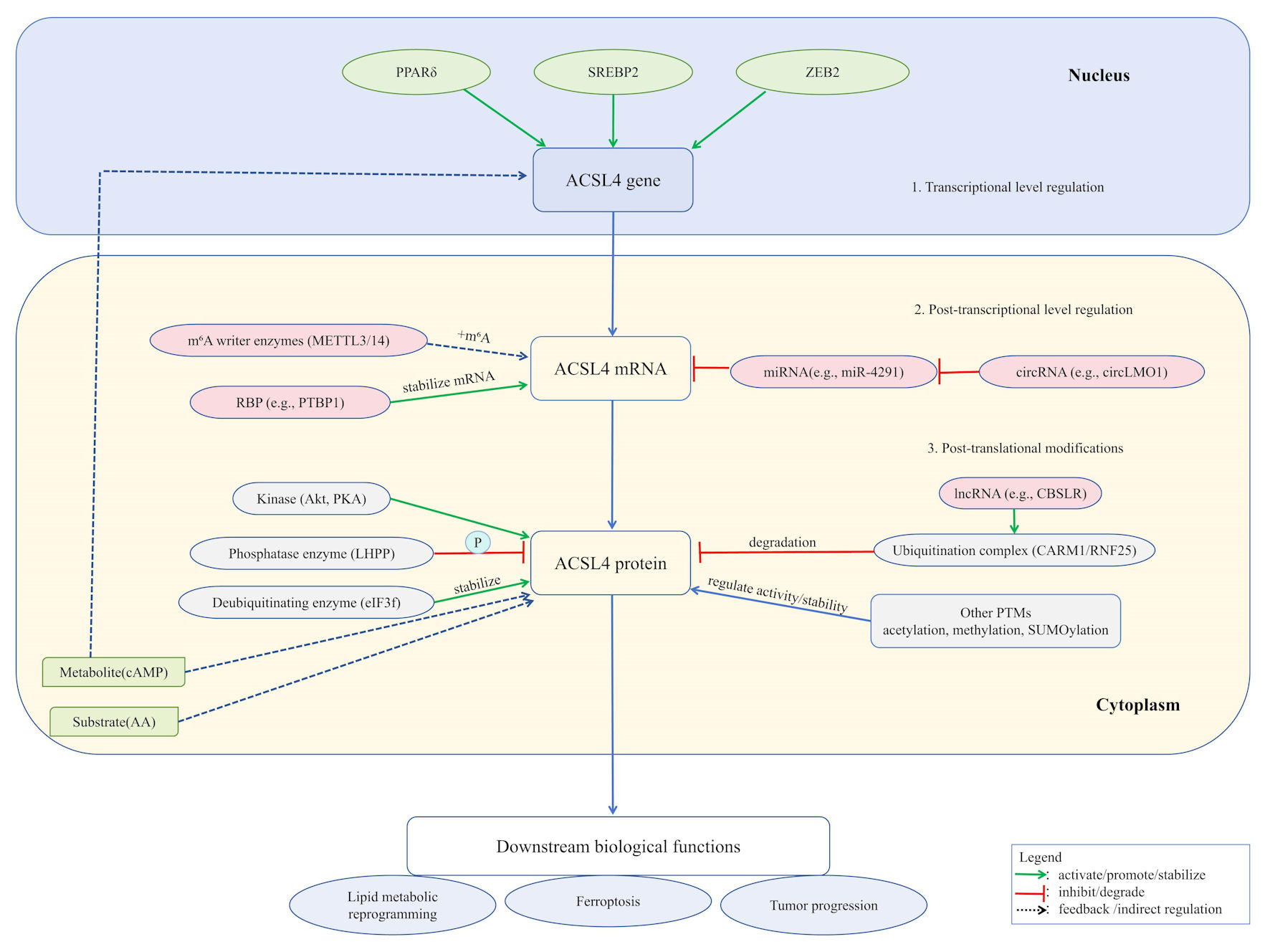
Figure 1. ACSL4 expression and function are subject to sophisticated, multi-layered regulation. At the transcriptional level, factors including PPARδ, SREBP2, and ZEB2 directly govern its gene expression. Post-transcriptionally, mRNA stability is enhanced by m6A modification (catalyzed by METTL3/14 complex) and RNA-binding proteins such as PTBP1, while non-coding RNAs (e.g., miR-4291 and circLMO1) exert regulatory effects through the ceRNA mechanism. At the post-translational level, ACSL4 protein activity is modulated by various modifications: ubiquitination (mediated by the CARM1/RNF25 complex and lncRNAs, such as CBSLR) and deubiquitination (e.g., eIF3f) regulate its stability; phosphorylation (e.g., Akt/PKA and LHPP) controls its function; additionally, modifications such as acetylation, methylation, and SUMOylation further fine-tune its activity and interactions. These integrated regulatory mechanisms collectively determine ACSL4’s biological roles in lipid metabolic reprogramming, ferroptosis, and tumor progression. ACSL4: acyl-CoA synthetase long-chain family member 4; PPARδ: peroxisome proliferator-activated receptor delta; SREBP2: sterol regulatory element-binding protein 2; ZEB2: zinc finger E-box binding homeobox 2; m6A: N6-methyladenosine; METTL3/14:methyltransferase-like 3/14; PTBP1: polypyrimidine tract-binding protein 1; Akt: protein kinase B; PKA: protein kinase A; LHPP: phospholysine phosphohistidine inorganic pyrophosphate phosphatase; eIF3f: eukaryotic translation initiation factor 3 subunit F; AA: arachidonic acid; cAMP: cyclic adenosine monophosphate; CARM1: coactivator-associated arginine methyltransferase 1; RNF25: ring finger protein 25.