| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Case Report
Volume 18, Number 3, June 2025, pages 152-158
Secondary Hyperparathyroidism in Primary Intestinal Lymphangiectasia: A Report of Four Cases
Dong Xue Zhanga, d, Kun Haob, d, Li Zhangc, Wen Bin Shenb, Tao Jiangb
aDepartment of Endocrinology, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China
bDepartment of Lymphatic Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China
cDepartment of Nuclear Medicine, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China
dThese authors contributed equally to this paper.
Corresponding Author: Tao Jiang and Wen Bin Shen, Department of Lymphatic Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, Chinaand
Manuscript submitted February 1, 2025, accepted May 12, 2025, published online June 4, 2025
Short title: Hyperparathyroidism in Lymphangiectasia
doi: https://doi.org/10.14740/gr2022
| Abstract | ▴Top |
Primary intestinal lymphangiectasia (PIL) is a rare disease characterized by the loss of lymphatic fluid in the intestinal lumen and is a known cause of protein-losing enteropathy (PLE). Although uncommon, few cases of secondary hyperparathyroidism (SHPT) have been reported in patients with PIL. This study summarizes the characteristics of four cases diagnosed with PIL. Notably, all cases were confirmed to have hyperparathyroidism secondary to vitamin D deficiency and hypocalcemia. Recurrent diarrhea and limb convulsions were also observed in all patients, with one patient diagnosed with osteoporosis. Simultaneously, hypomagnesemia was detected in three cases. Treatment with vitamin D and calcium supplements relieved symptoms, elevated serum calcium levels, and decreased parathyroid hormone (PTH) levels. In patients with PIL, evaluation of 25-hydroxyvitamin D, calcium, and PTH levels is crucial. Bone diseases should be considered in patients with SHPT, and appropriate vitamin D3 and calcium supplementation is highly recommended.
Keywords: Secondary hyperparathyroidism; Calcium; Vitamin D; Magnesium; Primary intestinal lymphangiectasia
| Introduction | ▴Top |
Protein-losing enteropathy (PLE) is characterized by excessive protein loss through the gastrointestinal tract [1], commonly due to lymphatic abnormalities or mucosal injury [1]. Although rare, primary intestinal lymphangiectasia (PIL) is a significant cause of PLE [2] and typically arises without an identifiable underlying condition. PIL is usually diagnosed during childhood and is rare in adults [3]. Meanwhile, IL can occur secondary to other conditions, including heart surgery and liver cirrhosis, which is defined as secondary intestinal lymphangiectasia (SIL) [4].
Low 25-hydroxyvitamin D (25(OH)D) levels have been observed in PLE [5]. Hypocalcemia is also relatively common in both PLE [1] and PIL [6]; however, only a few case reports on PIL [6, 7] have focused on secondary hyperparathyroidism (SHPT) resulting from hypocalcemia and vitamin D deficiency. Parathyroid hormone (PTH), secreted by the parathyroid gland, regulates the homeostasis of calcium and phosphorus by promoting renal calcium retention, enhancing intestinal absorption, and increasing skeletal resorption. PTH secretion is mediated by calcium-sensing receptors (CaSRs). Hypocalcemia weakens the inhibition effect of CaSR on PTH release [8]. Additionally, vitamin D binding with vitamin D receptor (VDR) also decreases PTH synthesis. Serum phosphate modulates PTH synthesis by influencing the concentration of fibroblast growth factor 23 (FGF-23) [9]. Thus, SHPT may result from oversecretion of PTH due to hypocalcemia, vitamin D deficiency, or hyperphosphatemia. SHPT has been found to be associated with increased bone turnover and lower bone density and may also impair the function of multiple extra-skeletal systems [10].
We report on SHPT and bone metabolism in four patients with PIL and summarize their clinical characteristics to explore potential management strategies for SHPT in the context of PIL.
| Case Reports | ▴Top |
Four patients were included in this study. Ethical approval was obtained from the Ethics Committee of Beijing Shijitan Hospital. Written informed consent was provided by the adult patient and guardians of all pediatric patients. All clinical tests were conducted following the Declaration of Helsinki guidelines.
Case 1
A 20-year-old woman presented with a 3-year history of a distended left leg and recurrent diarrhea accompanied by limb convulsions. She was admitted to our hospital and diagnosed with PIL. She underwent partial resection of the duodenum and small intestine 2 years ago. Postoperatively, failure to consume a low-fat diet resulted in more frequent diarrhea and limb convulsions.
The patient exhibited significantly reduced levels of total protein (31.4 g/L), albumin (16.6 g/L), globulin (14.8 g/L), and lymphocytes (0.35 × 109/L). Anemia (91 g/L) was also observed on admission. Fecal analysis revealed fat globules, and malabsorption syndrome was subsequently diagnosed. Protein loss from the intestine was detected using 99mTc-human serum albumin (HSA) scintigraphy (Fig. 1a). After excluding other secondary causes, PIL was diagnosed by gastroscopy.
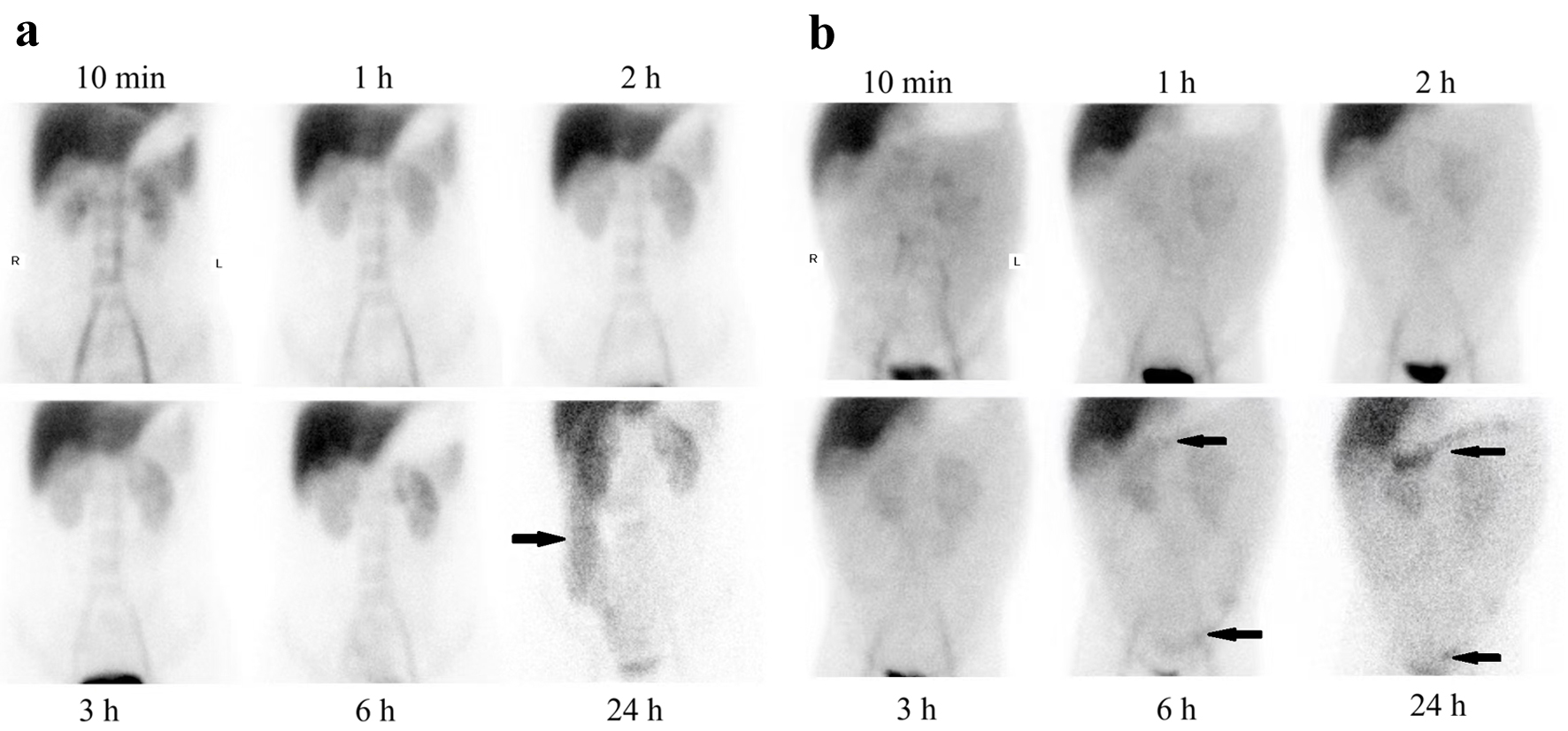 Click for large image | Figure 1. 99mTc-HSA scintigraphy in two patients. Protein was lost from intestine (black arrowheads) in case 1 (a) and case 4 (b). HAS: human serum albumin. |
A depressed total serum calcium (1.01 mmol/L) was observed even after adjusting for albumin (1.52 mmol/L). Other atypical blood results were phosphate 0.38 mmol/L (hypophosphatemia), magnesium 0.45 mmol/L (hypomagnesemia), and potassium 3.15 mmol/L (hypokalemia). The urine calcium was 6.34 mmol/24 h and within the normal range when the adjusted serum calcium level increased to the normal range (2.31 mmol/L). Before vitamin D supplementation, the level of 25(OH)D was below the lower limit of detection (< 3 ng/mL), indicating vitamin D deficiency. The intact PTH (iPTH) was 238.10 pg/mL (normal range, 12 - 88 pg/mL) (Table 1). Thus, elevated PTH levels, along with decreased concentrations of 25(OH)D and serum calcium, led to the diagnosis of SHPT.
 Click to view | Table 1. Biochemical Indicators in Patients |
Multiple vitamins were intravenously infused daily, including 5 µg of vitamin D2 and 2 g of calcium gluconate (containing 180 mg of elemental calcium). The adjusted calcium fluctuated between 1.49 and 1.96 mmol/L. Subsequently, an additional daily oral 2,250 mg of calcium carbonate (containing 900 mg of elemental calcium) was administered, ensuring blood calcium increased to 2.08 mmol/L and serum phosphorus to 0.78 mmol/L. Subsequently, blood magnesium levels were normalized. The patient received total parenteral nutritional (TPN) support, along with supplementation of lipid emulsion, amino acids, glucose, potassium, sodium, chloride, calcium, phosphorus, magnesium, water-soluble vitamins, fat-soluble vitamins, multi-trace elements, and water. A total duodenectomy, partial gastrectomy, choledochojejunostomy, pancreatojejunostomy, and gastrojejunostomy procedures were subsequently conducted. PIL was pathologically confirmed after surgery (Fig. 2a). Two months after surgery, in addition to a low-fat diet, the albumin and adjusted calcium concentrations increased to 35.3 g/L and 2.16 mmol/L, respectively.
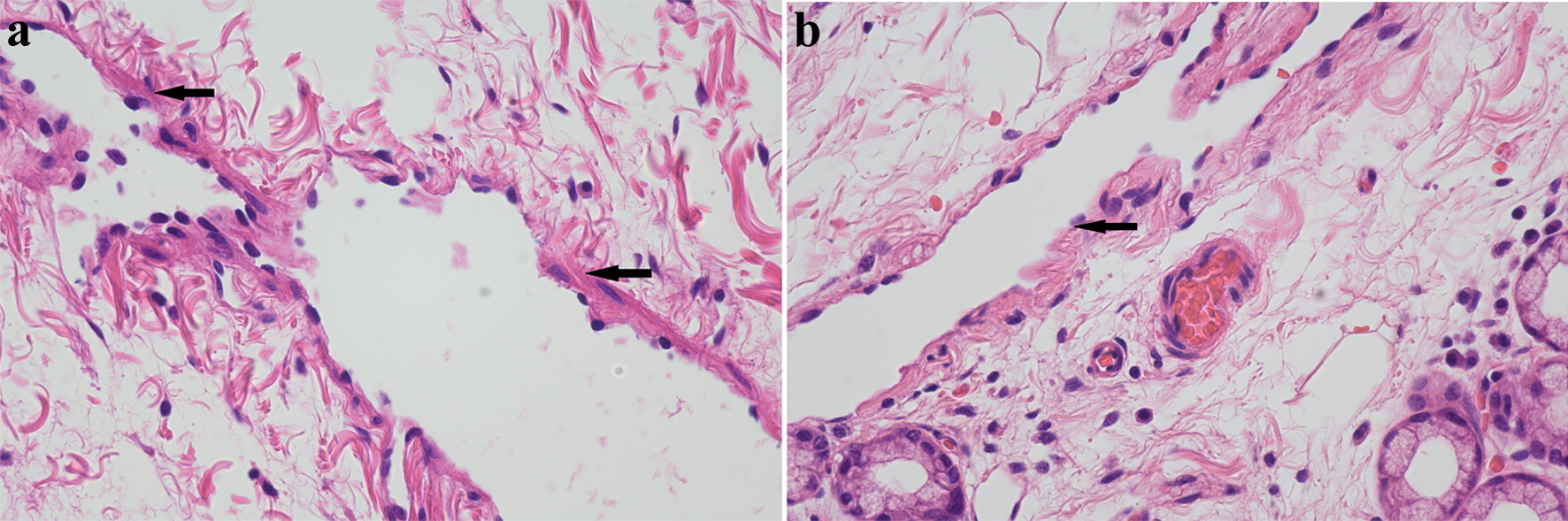 Click for large image | Figure 2. H&E staining of pathological sections. H&E staining (× 400) showed intestine lymphangiectasia (black arrowheads) in case 1 (a) and case 2 (b). H&E: hematoxylin and eosin. |
Case 2
A 17-year-old man was admitted to our hospital with a 10-year history of edema in the lower extremities and face and intermittent diarrhea that began 4 years before presentation. Over the last 2 years, he also developed lower back pain. Before admission, osteoporosis was diagnosed at a local hospital based on lower bone mineral density (BMD) derived from a dual-energy X-ray absorptiometry (DEXA) scan (the report cannot be provided).
We found decreased concentrations of total protein (37.3 g/L) and albumin (16.2 g/L), lymphocytes (0.5 × 109/L), but normal globulin (21.1 g/L). Protein loss from the intestine was confirmed by 99mTc-HSA scintigraphy, indicating PLE. While excluding other secondary factors, PIL was pathologically confirmed in the tissue specimen obtained during the lesional duodenectomy (Fig. 2b).
The patient exhibited severe hypocalcemia (1.03 mmol/L), hypophosphatemia (0.79 mmol/L), and hypomagnesemia (0.47 mmol/L). The iPTH level was elevated at 230.4 pg/mL. Notably, SHPT was diagnosed in the patient. Alkaline phosphatase (ALP) level (311 U/L; normal range, 40 - 150 U/L) was also elevated (Table 1).
Multiple vitamins containing 5 µg vitamin D2 and 2 g calcium gluconate (containing 180 mg of elemental calcium) were intravenously infused daily, and TPN was administered before and 3 weeks after surgery. Three weeks post-surgery, the serum calcium level increased to 2.02 mmol/L, PTH decreased to 202.1 pg/mL, and serum phosphorus and magnesium levels were normalized. After 1.5 years, the DEXA scan indicated that the osteoporosis had resolved. At the lumbar spine, bone density was 1.199 g/cm2 with a z-value of 1.1. Bone density at the femoral necks was 0.856 g/cm2, with a z-value of -1.2 standard deviation (SD) (> -2.0 SD).
Case 3
A 16-year-old girl was hospitalized with a 9-month history of generalized numbness and a 3-month history of recurrent diarrhea. Two months prior to admission, the patient was found to have hypocalcemia and hypoalbuminemia at a medical college hospital in China. Although her symptoms improved with a low-fat diet and calcium supplementation, the underlying cause remained unclear despite thorough evaluation.
The patient was admitted to our hospital for further evaluation. On admission, laboratory tests revealed lymphopenia (lymphocyte count: 0.4 × 109/L), hypoalbuminemia (16.1 g/L), hypoglobulinemia (13.1 g/L), and anemia (hemoglobin: 92 g/L). After excluding other potential causes, colonoscopy revealed intestinal lymphangiectasia in the terminal ileum. The diagnosis was confirmed by histopathological examination.
Blood tests revealed hypocalcemia (1.36 mmol/L) and hypomagnesemia (0.52 mmol/L). The serum phosphorus levels were normal. However, urine tests revealed normal calcium and low phosphorous levels in the urine (3.11 mmol/24 h; normal range: 2.05 - 7.5 mmol/24 h and 1.43 mmol/24 h, normal range: 14.0 - 41.98 mmol/24 h, respectively). Elevated iPTH levels (202.50 pg/mL) were also revealed (Table 1). Based on these findings, SHPT was confirmed.
In addition to the low-fat diet, albumin and 2 g calcium gluconate (containing 180 mg of elemental calcium) were intravenously infused daily. Subsequently, the patient’s symptoms were resolved. Six years later, the albumin and calcium levels increased to 36.9 g/L and 2.18 mmol/L, respectively. Phosphorus and magnesium levels were normal.
Case 4
A 16-year-old boy with diarrhea and abdominal distension was admitted to our hospital. At the age of 4, he was diagnosed with mesenteric lymphangioma and underwent bowel resection at a local hospital. However, his ascites and diarrhea persisted postoperatively. While a low-fat diet alleviated the diarrhea, it had no effect on the ascites. Gastrointestinal endoscopy revealed no abnormal findings.
Blood tests showed decreased levels of total protein (50 g/L), albumin (34.8 g/L), and globulin (15.7 g/L). Intestinal protein loss was confirmed by 99mTc-HSA scintigraphy, leading to the diagnosis of PLE. However, the exact location of the leak could not be identified (Fig. 1b). Plain computed tomography (CT) suggested possible dilation of the intestinal lymphatic vessels. Contrast-enhanced CT was not performed because the patient was allergic to iodine. Excluding other diseases, PLE in this patient may have resulted from PIL, although pathological exam was rejected.
Notably, a low lymphocyte count (0.63 × 109/L) was also detected. Electrolyte values were normal, except for mild hypocalcemia (2.08 mmol/L, normal range: 2.5 - 3.0 mmol/L). Vitamin D deficiency was confirmed based on a 25(OH)D detection (3 ng/mL). The iPTH level was elevated to 302.90 pg/mL, confirming the diagnosis of SHPT (Table 1). Additionally, bone turnover markers (BTMs) were abnormal: β-crosslaps (β-CTX) level was elevated to 2.120 ng/mL (normal value: < 0.704 ng/mL), total type 1 collagen amino terminal elongation peptide (tP1NP) was 270.90 ng/mL (normal range: 20 - 76 ng/mL), and N-terminal osteocalcin (N-MID) was 66.13 ng/mL (normal range: 11 - 46 ng/mL).
Based on these findings, a strict low-fat diet, without vitamin D or calcium supplementation, was initiated. Lymphoscintigraphy revealed thoracic duct obstruction, likely contributing to lymphatic vessel dilation. Surgical release of the end thoracic duct was performed to alleviate pressure on the intestinal lymph. As a result, both ascites and diarrhea resolved, and albumin and calcium levels increased to 31.7 g/L and 2.34 mmol/L, respectively.
| Discussion | ▴Top |
Besides the clinical manifestations, PLE can be confirmed by measuring alpha 1-antitrypsin levels in stool or through Tc-99m HSA [1, 11]. Alpha 1-antitrypsin is a serum glycoprotein with the same molecular size as albumin. It is resistant to degradation by intestinal proteases and is neither actively secreted nor absorbed in the digestive tract. Elevated fecal concentrations or increased clearance of alpha 1-antitrypsin can be used to assess the gastrointestinal protein loss [12]. A 24-h 99mTc-HSA scan provides reliable imaging for PLE diagnosis. The presence of tracer exudation into the gut confirms the condition. Furthermore, 99mTc-HSA can be used to determine the location of protein leakages [13, 14]. In the present cases, PLE was diagnosed based on 99mTc-HSA detection and typical clinical symptoms. The diagnostic criteria for PIL have been previously proposed as follows: 1) typical clinical symptoms; 2) hypoglobulinemia and hypoalbuminemia; 3) lymphocytopenia; 4) pathological diagnosis during endoscopy or surgery; and 5) imaging evidence for intestinal protein loss [15, 16]. Pathological diagnosis and imaging evidence are considered essential conditions [6, 17]. In this report, SHPT due to hypocalcemia and vitamin D deficiency was identified in the patients with PIL. PIL is a significant cause of PLE.
Hypovitaminosis D has been reported in dogs with PLE caused by either PIL or inflammatory bowel disease with SIL. In that study, of 43 dogs, hypovitaminosis D, but not albumin level, was associated with poor outcomes [18]. In humans, low vitamin D levels have similarly been linked to increased all-cause mortality [19]. Notably, no correlation was found between 25(OH)D3 levels and albumin concentration, suggesting that vitamin D-binding protein loss is not the primary driver of hypovitaminosis D in PLE [18]. In patients with IL, fat-soluble vitamins, such as vitamin D, are also lost from the intestine along with proteins, lymphocytes, and lipids [20]. All four patients in this report exhibited hypocalcemia, a frequent consequence of vitamin D deficiency in individuals with PIL or PLE. Tetany, a classic manifestation of hypocalcemia, was observed in our cases and has been reported in both children [21] and adults [22]. Additionally, hypocalcemic seizures have also been documented in infants with PIL [23]. In another animal study, a dog was diagnosed with lymphangiectasia after severe seizures from hypocalcemia [24].
In patients with vitamin D deficiency or hypocalcemia, PTH levels are increased to maintain calcium homeostasis, a condition defined as SHPT. SHPT was confirmed in all four of our patients. It typically arises in the context of reduced vitamin D levels, hypocalcemia, or hyperphosphatemia [8]. Low serum calcium concentrations reduce activation of the CaSR on parathyroid cells, thereby stimulating PTH secretion [25]. Conversely, the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), inhibits PTH synthesis and secretion while simultaneously suppressing parathyroid cell proliferation [26]. Vitamin D deficiency activates parathyroid function, while 1,25(OH)2D3 upregulates CaSR expression, thereby regulating PTH secretion [26]. Several reports have documented elevated PTH levels in patients with PIL [6, 7, 22]. In all previously reported cases, including the four in this study, hypocalcemia and vitamin D deficiency appears to jointly stimulate PTH release. As in PIL, excessive PTH secretion is also observed in secondary IL. For example, the Fontan procedure is a known cause of secondary IL [27], and in a cohort of Fontan patients, SHPT due to vitamin D deficiency was more common among those with PLE [28].
By contrast, excessive PTH secretion can activate osteoclasts and mobilize calcium from the bone to the serum, decreasing BMD [29, 30]. In our report, elevated BTMs were observed in two cases. In case 1, ALP levels were markedly increased. In case 4, both β-crosslaps and total P1NP were elevated, indicating simultaneous activation of osteoblasts and osteoclasts. Although no evidence of BTMs was identified in the PubMed database, ALP levels were elevated to 1,990 IU/L in another patient diagnosed with PIL [7]. Additionally, in case 2, osteoporosis was diagnosed before hospitalization. Osteoporosis is characterized by reduced BMD. Malabsorption of calcium, phosphorus, and vitamin D leads to a high risk of osteoporosis [2, 7, 31-33]. In both adult [31] and pediatric patients[33], decreased bone density and increased fracture risk have been associated with gastrointestinal diseases [33]. However, only three case studies have reported on abnormal bone metabolism. In a 63-year-old woman, bone disease was the first clinical manifestation associated with PIL. Her T-score was as low as -2.5 SD. Additionally, the patient was diagnosed with osteomalacia due to vitamin D deficiency [7]. Subsequently, low bone density was observed in a 4-year-old child with PIL [21], and a 16-year-old Chinese girl with PIL developed osteoporosis and osteomalacia [6]. In our study, DEXA was not available for most patients, and osteomalacia was not evaluated. Similar to our findings, hypocalcemia, vitamin D deficiency, and SHPT have been observed in these described cases [6, 7, 21]. A corresponding increase in BMD occurs following adequate vitamin D and calcium supplementation [6]. Hence, it is necessary to recognize and manage bone metabolism in PIL [6], especially in patients with SHPT.
Hypomagnesemia was observed in three patients in our report. In a literature review of PIL, only 23 (8%) patients were reported to have hypomagnesemia, making it less common compared with hypocalcemia [34]. Malabsorption due to gastrointestinal diseases, which leads to excessive loss of magnesium, is a major cause of hypomagnesemia [35]. Hypomagnesemia and hypocalcemia have also been reported in dogs with PLE [36]. Decreased magnesium and calcium concentrations may be associated with vitamin D deficiency and abnormal PTH secretion [36]. Magnesium is required in several vital steps involved in vitamin D synthesis, such as 25-hydroxylase activation, 25(OH)2 D synthesis, and VDR expression [37, 38]. Moreover, in patients with chronic kidney disease, magnesium intake alone cannot elevate vitamin D concentration; however, simultaneous supplementation with magnesium and vitamin D may be more effective in treating vitamin D deficiency [38]. Simultaneously, magnesium intake can relieve hypocalcemia resulting from hypomagnesemia [39]. Moreover, vitamin D is active in magnesium absorption in the intestine [38]. In patients with diabetes mellitus, magnesium concentrations significantly increase after cholecalciferol supplementation [40]. Similarly, in patients undergoing hemodialysis, magnesium levels are correlated with 25(OH)D concentrations [38, 41]. Complex interactions may exist between PTH and magnesium. Insufficient or excessive magnesium impairs PTH secretion [39]. However, PTH activates magnesium reabsorption by the tubules [42]. In patients with primary hyperparathyroidism [42] and postmenopausal women [43], hypomagnesemia may contribute to bone loss by suppressing osteoblast activity, reducing 1,25(OH)2 D vitamin synthesis, and promoting osteoclast activation [42, 44]. Hence, hypomagnesemia may accelerate hypocalcemia, hypovitaminosis D, and bone loss in patients with PIL.
In our case study, vitamin D2 and calcium gluconate were administered intravenously to all patients, resulting in the resolution of hypocalcemia resolved in most cases. PTH levels also decreased after treatment; however, in case 1, hypocalcemia persisted until oral calcium carbonate supplementation was administered. In a previously reported case of PIL, 0.25 µg of calcitriol and 1.2 g of calcium were administered orally daily, leading to a reduction in PTH levels after 1 month [6]. In another case of PIL, multiple vitamins, including 400 IU of vitamin D2, were administered intravenously during the hospital stay. Additionally, 0.75 g of alfacalcidol was administered orally each day, and intramuscular vitamins were administered monthly after discharge [21]. These findings suggest that vitamin D and calcium supplementation can effectively correct hypocalcemia and SHPT in patients with PIL. Among available options, vitamin D3 is recommended over vitamin D2 for treating vitamin D deficiency [45]. A higher dose of oral vitamin D3 is often recommended for patients with malabsorption [45, 46]. Intravenous or intramuscular vitamin D administration should be suggested as a second-line treatment if oral replacement is insufficient [21]. Cholecalciferol (vitamin D3) is converted in the liver into calcifediol (25(OH)D3, also known as calcidiol), which is subsequently metabolized to calcitriol (1,25(OH)2D3) in kidney [45]. In patients with hypovitaminosis D, daily supplementation with 800 - 2,000 IU of cholecalciferol is highly recommended [47]. An initial loading dose, such as daily 5,000 IU for 1 - 2 months, a single dose of 60,000 - 150,000 IU, or calcifediol 20 - 40 µg per day for 20 - 30 days, was suggested in patients with serum 25(OH)D < 10 ng/mL [47]. Some experts suggest that calcidiol may be more effective in treating vitamin D deficiency in patients with malabsorption or digestive diseases [45, 48]. However, owing to limited clinical evidence, calcidiol is not routinely recommended for treating vitamin D deficiency in clinical practice [45]. Some professionals hypothesize that the intake of active vitamin D compounds, such as calcitriol, is effective in patients with vitamin D deficiency [49]. However, active vitamin D has a short half-life and is associated with a high risk of hypercalciuria and hypercalcemia. Thus, it is not recommended to treat vitamin D deficiency [45, 49, 50]. Additionally, 800 - 1,000 mg calcium daily is recommended, accompanied by vitamin D supplementation. However, in our case, vitamin D and calcium supplementation was inadequate. Therefore, higher doses of oral vitamin D3 and adequate calcium may be the first choice for addressing vitamin D deficiency and SHPT in patients with PIL.
Conclusion
Decreased serum 25(OH)D and calcium levels, along with elevated PTH levels, have been observed in some patients with PLE. These patients were diagnosed with hyperparathyroidism secondary to vitamin D deficiency and hypocalcemia. Compensatory PTH secretion promotes active vitamin D synthesis and normalizes calcium and phosphorus metabolism. However, excessive PTH secretion may contribute to osteoporosis and osteomalacia in some cases of PIL. Notably, hypomagnesemia may accelerate this process. Therefore, measurement of 25(OH)D, calcium, and PTH concentrations is recommended in patients with PLE, especially those presenting with diarrhea and PIL. Additionally, patients with SHPT should be monitored for potential bone diseases. Adequate vitamin D3 and calcium supplementation can effectively alleviate metabolic disorders. Overall, early proper management may prevent SHPT and bone disorders in patients with PLE, particularly in those with PIL.
Learning points
SHPT was confirmed in patients with PIL.
Hypomagnesemia, osteoporosis, and abnormal bone turnover were detected.
Proper management of SHPT in PIL is crucial.
Acknowledgments
None to declare.
Financial Disclosure
This research was funded by Youth Fund Project, National Natural Science Foundation of China (No. 82200872 Dong Xue Zhang).
Conflict of Interest
The authors do not have any financial interests or relationships to this article.
Informed Consent
We have obtained written consent forms from the patient (case 1) and patients’ legal guardians (cases 2, 3, and 4) to publish the case.
Author Contributions
Dong Xue Zhang: conceptualization, data collection, and writing - original draft preparation. Kun Hao: conceptualization, data collection, and writing - original draft preparation. Li Zhang: data and figures collection and editing. Tao Jiang: conceptualization, supervision, and writing - reviewing and editing. Wen Bin Shen: conceptualization, supervision, and writing - reviewing and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ALP: alkaline phosphatase; Ca: calcium; DEXA: dual-energy X-ray absorptiometry; iPTH: intact parathyroid hormone; Mg: magnesium; 25(OH)D: 25-hydroxyvitamin D; P: phosphate; PIL: primary intestinal lymphangiectasia; PLE: protein-losing enteropathy; SHPT: secondary hyperparathyroidism; SIL: secondary intestinal lymphangiectasia; TPN: total parenteral nutrition; tP1NP: total type collagen amino terminal elongation peptide; U-Ca: calcium in urine; U-P: phosphate in urine
| References | ▴Top |
- Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169(10):1179-1185.
doi pubmed - Kwon Y, Kim MJ. The update of treatment for primary intestinal lymphangiectasia. Pediatr Gastroenterol Hepatol Nutr. 2021;24(5):413-422.
doi pubmed - Huber R, Semmler G, Mayr A, Offner F, Datz C. Primary intestinal lymphangiectasia in an adult patient: A case report and review of literature. World J Gastroenterol. 2020;26(48):7707-7718.
doi pubmed - Wilkinson P, Pinto B, Senior JR. Reversible protein-losing enteropathy with intestinal lymphangiectasia secondary to chronic constrictive pericarditis. N Engl J Med. 1965;273(22):1178-1181.
doi pubmed - Jones C, Jablonski SA, Petroff BK, Langlois DK. Relationship between serum magnesium, calcium, and parathyroid concentrations in dogs with abnormally low serum 25-hydroxyvitamin D concentration and chronic or protein-losing enteropathy. J Vet Intern Med. 2023;37(1):101-109.
doi pubmed - Li XP, Shen WB, Long MQ, Meng XW, Lian XL, Yu M. Osteomalacia and osteoporosis associated with primary intestinal lymphangiectasis. Chin Med J (Engl). 2012;125(10):1836-1838.
pubmed - Sahli H, Ben Mbarek R, Elleuch M, Azzouz D, Meddeb N, Cheour E, Azzouz MM, et al. Osteomalacia in a patient with primary intestinal lymphangiectasis (Waldmann's disease). Joint Bone Spine. 2008;75(1):73-75.
doi pubmed - Stack BC, Jr. Secondary Hyperparathyroidism. Otolaryngol Clin North Am. 2024;57(1):99-110.
doi pubmed - Cozzolino M, Galassi A, Conte F, Mangano M, Di Lullo L, Bellasi A. Treatment of secondary hyperparathyroidism: the clinical utility of etelcalcetide. Ther Clin Risk Manag. 2017;13:679-689.
doi pubmed - Komaba H, Kakuta T, Fukagawa M. Management of secondary hyperparathyroidism: how and why? Clin Exp Nephrol. 2017;21(Suppl 1):37-45.
doi pubmed - Kobayashi Y, Shimojima Y, Kondo Y, Takamatsu R, Miyazaki D, Kishida D, Sekijima Y, et al. Protein-losing gastroenteropathy related to mixed connective tissue disease: a case report of a successful outcome and literature review. Intern Med. 2017;56(15):2057-2062.
doi pubmed - Ozen A, Lenardo MJ. Protein-Losing Enteropathy. N Engl J Med. 2023;389(8):733-748.
doi pubmed - Pascal P, Malloizel J, Lairez O, de Volontat MD, Bournet B. Primary intestinal lymphangiectasia: diagnostic accuracy of 99mTc-labeled human serum albumin Nanocolloid SPECT/CT before biopsy. Clin Nucl Med. 2021;46(1):e34-e35.
doi pubmed - Chiu NT, Lee BF, Hwang SJ, Chang JM, Liu GC, Yu HS. Protein-losing enteropathy: diagnosis with (99m)Tc-labeled human serum albumin scintigraphy. Radiology. 2001;219(1):86-90.
doi pubmed - Wen Z, Tong G, Liu Y, Meeks JK, Ma D, Yang J. The lymphoscintigraphic manifestation of (99m)Tc-dextran lymphatic imaging in primary intestinal lymphangiectasia. Nucl Med Commun. 2014;35(5):493-500.
doi pubmed - Lopez RN, Day AS. Primary intestinal lymphangiectasia in children: A review. J Paediatr Child Health. 2020;56(11):1719-1723.
doi pubmed - Zhu LH, Cai XJ, Mou YP, Zhu YP, Wang SB, Wu JG. Partial enterectomy: treatment for primary intestinal lymphangiectasia in four cases. Chin Med J (Engl). 2010;123(6):760-764.
pubmed - Allenspach K, Rizzo J, Jergens AE, Chang YM. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet Res. 2017;13(1):96.
doi pubmed - Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91-100.
doi pubmed - Ingle SB, Hinge Ingle CR. Primary intestinal lymphangiectasia: Minireview. World J Clin Cases. 2014;2(10):528-533.
doi pubmed - Lu YY, Wu JF, Ni YH, Peng SS, Shun CT, Chang MH. Hypocalcemia and tetany caused by vitamin D deficiency in a child with intestinal lymphangiectasia. J Formos Med Assoc. 2009;108(10):814-818.
doi pubmed - Cao Y, Feng XH, Ni HX. Primary intestinal lymphangiectasia presenting as limb convulsions: A case report. World J Clin Cases. 2022;10(18):6234-6240.
doi pubmed - Van Biervliet S, Velde SV, Robberecht E, Van Winckel M. Hypocalcaemic seizures: sign of intestinal disease? Acta Gastroenterol Belg. 2007;70(2):243-244.
pubmed - Whitehead J, Quimby J, Bayliss D. Seizures associated with hypocalcemia in a Yorkshire terrier with protein-losing enteropathy. J Am Anim Hosp Assoc. 2015;51(6):380-384.
doi pubmed - Hannan FM, Kallay E, Chang W, Brandi ML, Thakker RV. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol. 2018;15(1):33-51.
doi pubmed - Xiang Z, Wang M, Miao C, Jin D, Wang H. Mechanism of calcitriol regulating parathyroid cells in secondary hyperparathyroidism. Front Pharmacol. 2022;13:1020858.
doi pubmed - Johnson JN, Driscoll DJ, O'Leary PW. Protein-losing enteropathy and the Fontan operation. Nutr Clin Pract. 2012;27(3):375-384.
doi pubmed - Holler F, Hannes T, Germund I, Emmel M, Hoyer-Kuhn H, Khalil M, Sreeram N, et al. Low serum 25-hydroxyvitamin D levels and secondary hyperparathyroidism in Fontan patients. Cardiol Young. 2016;26(5):876-884.
doi pubmed - Kim H, Oh B, Park-Min KH. Regulation of osteoclast differentiation and activity by lipid metabolism. Cells. 2021;10(1):89.
doi pubmed - Fischer LE, Moreno-Garcia F, Tran R, Harmon A, Little C, Domingue G, Stewart K, et al. Prevalence and risk factors for secondary hyperparathyroidism (SHPT) in patients undergoing bariatric surgery. Surg Endosc. 2023;37(10):8019-8028.
doi pubmed - Oh HJ, Ryu KH, Park BJ, Yoon BH. Osteoporosis and osteoporotic fractures in gastrointestinal disease. J Bone Metab. 2018;25(4):213-217.
doi pubmed - Semrad CE. Bone mass and gastrointestinal disease. Ann N Y Acad Sci. 2000;904:564-570.
doi pubmed - Yang HR. Updates on bone health in children with gastrointestinal diseases. Ann Pediatr Endocrinol Metab. 2020;25(1):10-14.
doi pubmed - Feng H, Zou L, Zhai X, Zhang S, Li J. Hypomagnesemia in intestinal lymphangiectasia: a case report and review of the literature. BMC Gastroenterol. 2022;22(1):246.
doi pubmed - Flink EB. Magnesium deficiency. Etiology and clinical spectrum. Acta Med Scand Suppl. 1981;647:125-137.
doi pubmed - Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire terriers: five cases (1992-1998). J Am Vet Med Assoc. 2000;217(5):703-706.
doi pubmed - Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118(3):181-189.
doi pubmed - Matias P, Avila G, Ferreira AC, Laranjinha I, Ferreira A. Hypomagnesemia: a potential underlooked cause of persistent vitamin D deficiency in chronic kidney disease. Clin Kidney J. 2023;16(11):1776-1785.
doi pubmed - Fong J, Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician. 2012;58(2):158-162.
pubmed - Al-Daghri NM, Alkharfy KM, Khan N, Alfawaz HA, Al-Ajlan AS, Yakout SM, Alokail MS. Vitamin D supplementation and serum levels of magnesium and selenium in type 2 diabetes mellitus patients: gender dimorphic changes. Int J Vitam Nutr Res. 2014;84(1-2):27-34.
doi pubmed - Matias PJ, Laranjinha I, Avila G, Azevedo A, Jorge C, Ferreira C, Aires I, et al. Long-term cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac parameters. Semin Dial. 2023;36(1):29-36.
doi pubmed - Duger H, Ucan B, Caliskan M, Bostan H, Demirci T, Gul U, Cakal E, et al. Hypomagnesemia may be associated with symptomatic disease in patients with primary hyperparathyroidism. Endocrine. 2024;83(2):466-472.
doi pubmed - Okyay E, Ertugrul C, Acar B, Sisman AR, Onvural B, Ozaksoy D. Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas. 2013;76(4):320-325.
doi pubmed - Zofkova I, Nemcikova P, Matucha P. Trace elements and bone health. Clin Chem Lab Med. 2013;51(8):1555-1561.
doi pubmed - Pludowski P, Takacs I, Boyanov M, Belaya Z, Diaconu CC, Mokhort T, Zherdova N, et al. Clinical practice in the prevention, diagnosis and treatment of vitamin D deficiency: a central and Eastern European expert consensus statement. Nutrients. 2022;14(7):1483.
doi pubmed - Nielsen OH, Hansen TI, Gubatan JM, Jensen KB, Rejnmark L. Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterol. 2019;10(4):394-400.
doi pubmed - Bertoldo F, Cianferotti L, Di Monaco M, Falchetti A, Fassio A, Gatti D, Gennari L, et al. Definition, assessment, and management of vitamin D inadequacy: suggestions, recommendations, and warnings from the Italian society for osteoporosis, mineral metabolism and bone diseases (SIOMMMS). Nutrients. 2022;14(19):4148.
doi pubmed - Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29(8):1697-1711.
doi pubmed - Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153-165.
doi pubmed - Ramasamy I. Vitamin D metabolism and guidelines for vitamin D supplementation. Clin Biochem Rev. 2020;41(3):103-126.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.