| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 2, April 2025, pages 49-55
Blood Product Utilization in Thromboelastography-Aided Transfusion in Gastrointestinal Bleeding: A Single-Center Experience
Mohammad Abdulelaha, g, Aleezay Asgharb, Michael Sansaitc, Vida Rastegard, Danielle Walshe, Joshua Allgaierf, Nakul Ravikumarf
aDepartment of Internal Medicine, Baystate Medical Center, University of Massachusetts Chan Medical School, Springfield, MA 01199, USA
bDepartment of Pulmonary, Critical Care, Sleep, and Allergy, University of Illinois Chicago, Chicago, IL 60612, USA
cDepartment of Critical Care Medicine, Eden Medical Center, Castro Valley, CA 94546, USA
dDepartment of Medicine and Institute for Healthcare Delivery and Population Science, Baystate Medical Center, University of Massachusetts Chan Medical School, Springfield, MA 01199, USA
eDepartment of Critical Care Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, NY 11030, USA
fDepartment of Pulmonary & Critical Care Medicine, Baystate Medical Center, University of Massachusetts Chan Medical School, Springfield, MA 01199, USA
gCorresponding Author: Mohammad Abdulelah, Department of Internal Medicine, Baystate Medical Center, University of Massachusetts Chan Medical School, Springfield, MA 01199, USA
Manuscript submitted February 3, 2025, accepted March 29, 2025, published online April 11, 2025
Short title: TEG-Aided Transfusion in GIB
doi: https://doi.org/10.14740/gr2025
| Abstract | ▴Top |
Background: Gastrointestinal bleeding (GIB) is a common cause for intensive care unit (ICU) admissions and is associated with high mortality rates. Effective resuscitation is essential prior to definitive procedural intervention. Thromboelastography (TEG) assesses patients’ dynamic coagulation profiles and has been shown to reduce blood product usage and mortality in specific patient populations; however, its role in the management of GIB remains controversial.
Methods: We performed a retrospective study of patients who had TEG performed during resuscitation of GIB in the ICU between January 1, 2017 and December 31, 2020 at a single center. Patients were identified through ICD-10 codes and blood bank’s database.
Results: A cohort of 244 patients was identified, of which 18 were excluded. The cohort was mainly represented by White (72%, n = 162) males (65%, n = 147) with a mean age of 61 (standard deviation (SD) 14) years. Alcoholic liver disease (31%, n = 69) and esophageal varices (30%, n = 65) were the most common comorbidities. Mean nadir systolic blood pressure was 75 (SD 18) mm Hg. Mean nadir hemoglobin concentration was 6.5 (SD 1.7) g/dL. Patients received a median of 5 packed red blood cells (pRBC) (interquartile range (IQR) 5.8), 1 fresh frozen plasma (FFP) (IQR 2), and 0 platelets and cryoprecipitate units (IQR 1 and 0, respectively). The median ICU length of stay was 3 (IQR 3) days. The observed mortality rate was 39% (n = 88).
Conclusion: Although TEG may help reduce unnecessary blood product transfusions, its overall clinical benefit remains uncertain given the high mortality observed among patients with hemorrhagic shock secondary to GIB. Further studies are warranted to better evaluate the efficacy and clinical utility of TEG-guided transfusion strategies in this patient population.
Keywords: Gastrointestinal bleeding; Thromboelastography; Restrictive resuscitation; Hemorrhagic shock; Mortality
| Introduction | ▴Top |
Viscoelastic hemostatic assays (VHAs), such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM), are point-of-care hemostasis assessment devices that measure the viscoelastic changes that occur during hemostasis. They provide near real-time assessment of platelet interaction, coagulation cascade, and clot formation/dissolution, providing a dynamic description of coagulation parameters. TEG-guided transfusion protocols have gained popularity when resuscitating surgical and trauma patients [1]. In particular, TEG-guided resuscitation is extensively used in the care of preoperative and postoperative cardiac surgery patients [2, 3]. Their use is endorsed by multiple surgical guidelines, such as the Eastern Association for the Surgery of Trauma, which conditionally recommends using VHA-guided resuscitative efforts versus non-VHA strategies in critically ill patients with ongoing hemorrhage and concern for coagulopathy, with the ultimate aim of reducing the overall volume of transfused blood products [4]. However, it is important to note that the critically ill patient population in the index study was heterogenous, and included cardiac surgery patients whose postoperative care is distinct in its approach. Multiple studies have addressed transfusion outcomes in post-cardiac surgery patients, as well as those with cirrhosis, and have demonstrated reduced mortality and decreased blood product usage when blood product transfusion was guided by TEG parameters [5, 6]. For instance, a reduction in the use of blood products and about 50% lower 7-day mortality among patients with cirrhosis receiving TEG-guided resuscitation were previously reported; however, no significant differences were reported in bleeding rates or long-term mortality at 42 days [7]. To date, TEG-guided protocols have not been developed for non-surgical patients such as those encountered in the medical intensive care unit (ICU).
Gastrointestinal bleeding (GIB) is a common etiology of hemorrhage in non-surgical and non-trauma patients, with 150,000 annual hospital admissions and an associated mortality of 2-17% [8-11]. Following trauma, it is the second most common diagnosis that requires activation of massive transfusion protocol (MTP) [12]. However, restrictive strategies have been shown to significantly improve outcomes in patients with acute upper GIB when compared to more liberal resuscitation [13]. Yet, randomized clinical trials comparing standard-of-care resuscitation to factor-specific approaches, such as TEG-guided resuscitation, in specific disease phenotypes remain limited.
We conducted a descriptive study to assess the clinical outcomes in patients with hemodynamically unstable GIB who also had a TEG performed as part of their care. Given the limited availability of blood products, and volume overload and transfusion-related adverse effects associated with massive transfusion, we aimed to explore the clinical trajectories and outcomes among those who underwent TEG during life-threatening GIB.
| Materials and Methods | ▴Top |
We conducted a retrospective descriptive study of patients admitted to the medical ICU for hemorrhagic shock due to GIB and had TEG performed as part of their resuscitation. The study spanned a 48-month period, between January 1, 2017 and December 31, 2020, at Baystate Medical Center in Springfield in Western Massachusetts. The study protocol adhered to ethical guidelines and was approved by the hospital’s institutional review board as an exempt project (ID number 1776664-8).
We included patients > 18 years of age who had GIB requiring ICU admission. We identified patients with hemodynamically unstable GIB requiring ICU admission through the organization’s data warehouse using International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) code K92.2 reflecting GIB and Current Procedural Terminology (CPT) codes 99291 and 99292 indicating ICU level of care. The identified patient encounters were then cross-referenced with the blood bank’s TEG database. To decrease misclassification error due to inappropriate coding, a resident physician (post-graduate year 2) accessed the patient’s charts to ensure that the attending physician listed GIB as the principal diagnosis for ICU admission. Patients were excluded if the bleeding etiology was thought to be not within the gastrointestinal (GI) tract based on the admitting attending physicians’ impression and those admitted for trauma.
Data points were collected and managed using REDCap (Research Electronic Data Capture) data capture tools hosted at Tufts Clinical and Translational Science Institute [14]. Patient demographic data retrieved for the encounter of interest included age, sex, ethnicity, and body mass index (BMI). We also recorded nadir blood pressures (systolic and diastolic), highest and lowest heart rate, presenting and nadir hemoglobin and hematocrit, prothrombin time (PTT), international normalized ratio (INR), and platelet levels. Additionally, the highest recorded levels of creatinine, blood urea nitrogen (BUN), and lactate within 48 h of presentation were recorded as surrogates for end organ damage. Data regarding prior medical history were also obtained, and included alcoholic liver disease (ALD), fatty liver disease, viral hepatitis, peptic ulcer disease (PUD), varices, prior abdominal surgeries, malignancy, and proton pump inhibitor (PPI) and steroids use. We recorded outcomes including the total number of blood products administered within 48 h of presentation, use of vasopressor support, need for surgery on presentation, ICU length of stay (LOS), total hospital LOS, in-hospital mortality, and discharge destination. Blood products including packed red blood cells (pRBCs), cryoprecipitate, platelets, and fresh frozen plasma (FFP) were identified using the unique ID assigned to each transfused unit.
Descriptive statistics were carried out to offer a snapshot of essential features within the dataset including counts and percentages to describe the sample characteristics for categorical variables, while mean and standard deviation (SD) were used for continuous variables after testing for normality. Medians and interquartile ranges (IQRs) were used to describe non-normally distributed data.
| Results | ▴Top |
A total of 244 patients were identified, 18 (7.4%) of which were excluded based on the aforementioned criteria. A total of 226 patients were included in the final statistical analysis.
Our cohort mainly consisted of males (65%, n = 146), with a mean age of 61 (SD 14, range 22 - 99) years. The majority of the patients were White (72%, n = 162), followed by Hispanic (15%, n = 33). Most commonly noted baseline chronic comorbidities included ALD (31%, n = 69), history of esophageal varices (29%, n = 65), history of PUD (25%, n = 57), and prior history of abdominal surgery in the last 5 years (22%, n = 50). Notably, patients with metabolic dysfunction-associated liver disease (MALD) comprised a small percentage (6.6%, n = 15). About 28% (n = 63) of the cohort were on PPIs and 8% (n = 18) were on chronic steroid therapy (Table 1). The cohort’s mean nadir systolic blood pressure (SBP) within 48 h of admission to the medical ICU was 75 (SD 18) mm Hg, while the mean nadir diastolic blood pressure (DBP) was 39 (SD 12) mm Hg. The mean peak heart rate was 124 (SD 26) beats per minute. On admission to the medical ICU, the mean hemoglobin was 9.47 (SD 2.94) g/dL (normal 11.7 - 15.5), and 6.50 (SD 1.70) g/dL at nadir within 48 h of admission. Peak creatinine within 48 h was 2.1 (SD 1.62) mg/dL (normal 0.5 - 1). Peak serum lactate within 48 h was 5.9 (SD 5.5) mmol/L (normal 0.5 - 2.2). All disease groups were hemodynamically unstable on admission to the ICU. Furthermore, markers of end organ damage within 48 h of admission were also similar (Table 2). Vasopressor use was not available in 17 records. Twelve records were missing whether patients had prior diagnosis of esophageal varices or PUD. Steroid and PPI use was missing from five records. While history of abdominal surgery was not documented in three records.
 Click to view | Table 1. Patient Characteristics |
 Click to view | Table 2. Indicators of Hemodynamic Stability and Markers of End Organ Damage Across Different Disease Groups |
Patients received a median of 7 units of blood products (IQR 8) within the first 48 h until hemodynamic stability was achieved and a minimum hemoglobin of 7 was achieved. Patients received a median of 5 (IQR 5.8) pRBC and 1 (IQR 2) unit of FFP. Only 34% (n = 77) received platelet transfusion with a median of 1.0 (IQR 2) transfused units. Those with a history of PUD received a median of 6 (IQR 6) pRBCs, while those with a history of varices or ALD received 4 (IQR 5 and 4, respectively) units. The median number of transfused FFP units was 1 (IQR 2) for those with ALD, prior PUD, and prior esophageal varices. There was a median of 0 transfused platelet units for those with ALD, varices, and PUD (IQR 1, 1, and 0, respectively). Those who were on chronic PPI therapy prior to admission required a median of 6 (IQR 6.5) total units, while those who were not on PPI therapy required a total of 7 (IQR 8) total units.
Despite resuscitation, 65% (n = 147) of patients required vasopressor support. Seventy-two percent (n = 50) of those with ALD required vasopressor support, while 69% (n = 45) of those with esophageal varices and 60% (n = 34) of those with PUD did not. Overall, those who received vasopressor support received a median of 7 total blood products (IQR 8), while those who did not receive vasopressor support received a median of 5 (IQR 7) units.
Our cohort’s in-hospital mortality was 38.9% (n = 88), of which 41% (n = 36) passed away within the first 48 h of admission. The highest mortality was noted amongst those who have a prior history of esophageal varices, estimated around 38% (n = 30) followed by ALD at 36% (n = 32), and PUD at 21% (n = 16).
Median ICU LOS was 3 days (IQR 3). No difference was noted across the three disease groups in terms of ICU LOS. The median hospital LOS was 9 (IQR 14.75) days. Those with PUD had the longest hospital stay with a median of 10 (IQR 17) days when compared to those with history of esophageal varices or ALD at 5 (IQR 13) days and 6 (IQR 11) days, respectively (P = 0.001) (Fig. 1). Forty-one percent (n = 93) of patients required additional healthcare services upon discharge and 18.6% (n = 42) of patients were discharged home.
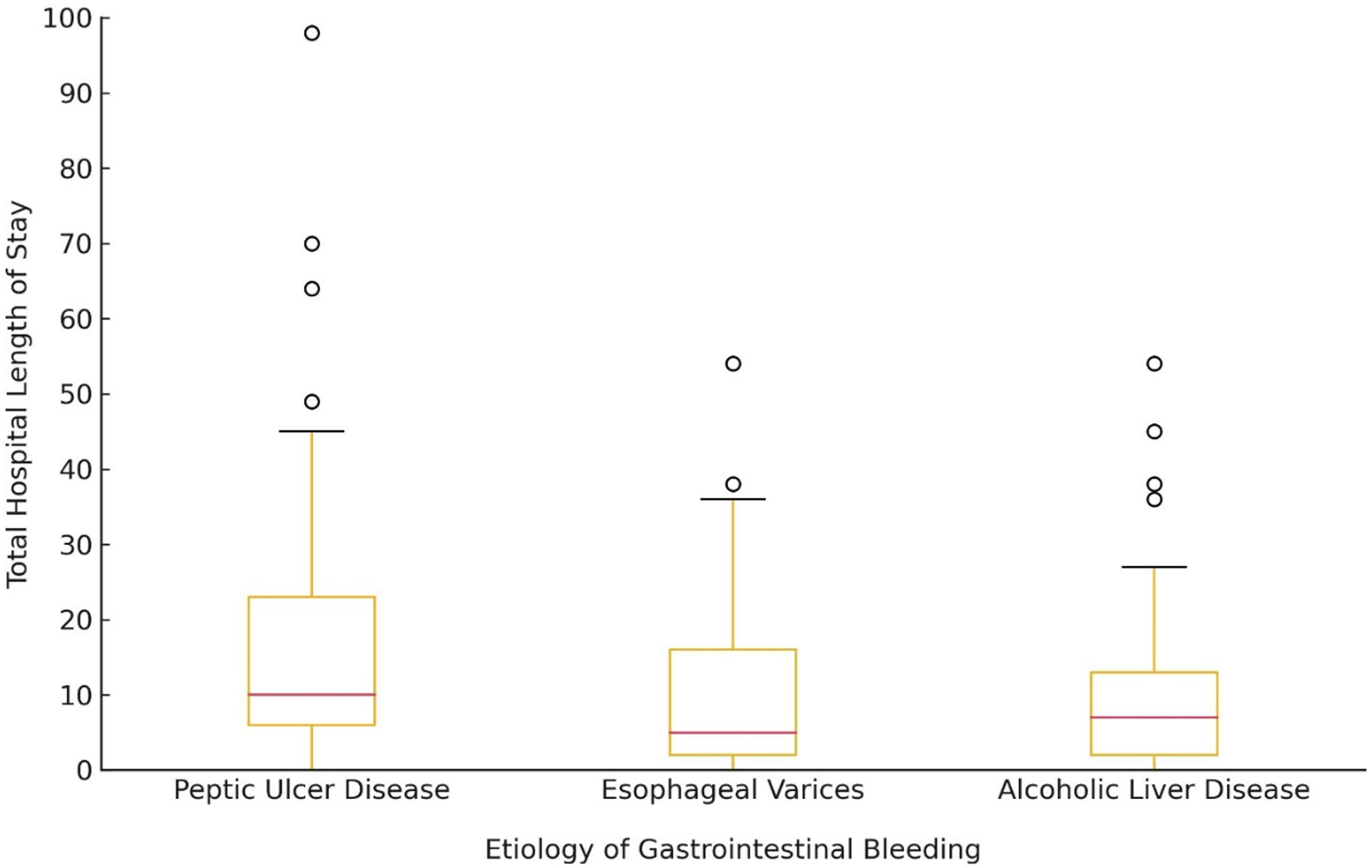 Click for large image | Figure 1. Box plot illustrating the distribution of total hospital length of stay (in days) for patients with different etiologies of gastrointestinal bleeding. The median (red line), interquartile range, and outliers are displayed. |
| Discussion | ▴Top |
We describe a cohort of patients with hemorrhagic shock due to GIB who had a TEG obtained as part of their workup. The transfusion patterns in our study were discordant from the commonly cited MTP. The MTP was originally introduced for traumatic hemorrhage and later carried over for non-traumatic etiologies of hemorrhagic shock despite lacking evidence [15]. On the one hand, massive transfusion is defined as 4 - 10 units of blood products over 24 h [16]. While on the other hand, MTP is defined as administering blood products as a fixed 1:1:1 ratio of pRBC/platelets/FFP to address potential coagulopathy associated with severe trauma [17]. Patients with non-traumatic and non-cirrhotic etiologies of hemorrhage often lack the hyperfibrinolysis and early coagulopathy that is often observed in traumatic hemorrhage which debates against the utility of MTP and suggests a potential role for TEG-guided resuscitation [18]. Upon further analysis of transfusion variation, most of the patients in our cohort did not receive platelet transfusions which potentially supports the hypothesis that obtaining a TEG leads to more specific transfusion patterns rather than empiric transfusion. Namely, utilizing TEG would lead to avoidance of unnecessary FFP and platelet transfusion, given that TEG parameters are not abnormal. Notably, there are no published randomized evidence supporting a specific transfusion threshold for platelets in patients with active GIB; however, based on expert opinion, a transfusion threshold of 50 × 109/L has been suggested [19, 20]. Thus, our findings raise the possibility that TEG-aided resuscitation of severe GIB could potentially lead to a decrement in overall blood product utilization, which is highly desired given the increase in mortality noted with larger transfusion volumes [21]. The deviation from MTP is of significant importance due to the potential association between the larger transfused volumes in MTP and the increased risks of transfusion-related acute lung injury and death, coupled with the limited availability of high-quality evidence for MTP in those with GIB [22]. Our findings were in alignment with a study conducted by Kumar et al, which described a statistically significant decrease in utilization of blood products when administering blood products to patients with non-variceal upper GIB in patients with cirrhosis when following TEG parameters [23]. However, it must be noted that only about 20% of all cases of upper GIB are attributed to cirrhosis and there are limited data regarding the utility of TEG-guided resuscitation in patients with non-cirrhotic GIB [24, 25]. Therefore, our study fills an important gap in the literature as our findings are more inclusive and included all patients with GIB. Nonetheless, our findings were discordant from a previously published study by Rizvi et al, which described an overall increase in blood product utilization in patients with GIB who were admitted to the ICU and resuscitated according to TEG parameters compared to a control arm [26]. Additionally, one institute’s experience with using TEG as part of standardized testing revealed that those who were resuscitated according to TEG parameters received more blood transfusions than non-TEG patients [27]. Using TEG scan could potentially augment targeted blood product use but current evidence from observational data is rife with confounders, including but not limited to severity of illness that may provoke use of TEG. Remarkably, TEG parameters have been identified to be more deranged in sicker patients calling into reconsideration the utility of TEG-guided resuscitation [28].
Notably, a high mortality rate of 39% was observed in our cohort which was concordant with the few published studies evaluating the use of TEG in patients with medical etiologies of hemorrhagic shock [7]. For instance, one retrospective analysis revealed a similar high mortality rate of 34% when patients with GIB were resuscitated according to TEG parameters compared to 9.8% when standard coagulation parameters were followed [26]. It is essential to note that most of our patients were hemodynamically unstable, presenting with class III-IV hemorrhagic shock per the American College of Surgeons’ Advanced Trauma Life Support (ATLS) classification of hypovolemic shock [29]. The higher mortality rate in our cohort of GIB may also be partially attributed to our institution’s role as a safety-net healthcare system, which provides care for those with minimal access to healthcare, where those with stable GIB are commonly managed at our intermediate care unit rather than the ICU. This can also reflect the higher transfusion requirement than the average of 2.4 units of pRBC that has been previously described in the literature [30]. The clinical complexity of our cohort was also evidenced by the fact that 70% of our cohort required vasopressor support to maintain hemodynamics while undergoing resuscitation, which is significantly higher than previously studied cohorts for similar presentations [26]. However, it must be noted that randomized clinical trials failed to reveal a significant decrease in 28-day mortality rates when vasopressors are administered to those with GIB [31]. Our findings of severely deranged hemodynamics, high blood product usage, and the frequent use of vasopressor support raise the question of whether physicians opted to obtain a TEG in anticipation of a challenging clinical course due to the severity of the patients’ presentations.
Our findings also highlight a variation in hospital and healthcare resource use and burden. Our findings suggest possible discordance in total hospital stay but not ICU LOS across the varying etiologies of GIB, where we observed that those with PUD have the highest total LOS, which supports previously published literature [32]. This finding could reflect the important nuances of post-ICU care in terms of both medical management and resource allocations. This is of particular relevance to hospital administrators to address to decrease the overall healthcare burden and resource utilization. Moreover, the healthcare burden of those with GIB extended beyond the index hospitalization, where the majority of survivors in our study required additional healthcare services upon discharge.
Our study has several limitations. Firstly, data regarding fluid resuscitation and TEG parameters, and to what degree such parameters were followed during resuscitation, were not evaluated. Secondly, there was no control group and therefore, TEG-related outcomes could not be compared to the current standard of care. Thirdly, being a retrospective study lends itself to both confounding and selection bias. Within this context, confounding factors such as severity of bleeding were not accounted for. Data regarding whether any endoscopic or radiological interventions were performed were not obtained as our study focused on acute resuscitation and management of GIB. Additionally, our inclusion criteria of hemorrhagic shock were based on ICD-10 coding and physician’s clinical judgment supported by hemodynamic and laboratory results, which on the one hand reflects daily clinical practice, but can also be subject to human error. Lastly, the unavailability of anticoagulation status in our study is a major drawback. However, clinical judgement regarding appropriate reversal, rather than relying on laboratory workup, depicts a more realistic approach to practice.
Conclusion
TEG-aided resuscitation might lead to a deviation from fixed-ratio blood transfusion protocols in those with GIB. Our findings are hypothesis-generating and underscore the potential utility of TEG-aided resuscitation in those with hemorrhagic shock due to GIB without evidence of coagulopathy. However, caution must be exercised when TEG is performed as we noticed a high mortality rate. Further research is needed to ensure appropriate safety and viability of following TEG and to guide institutional policies regarding protocolized use of TEG-guided resuscitation.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Patient consent was not obtained because the retrospective study utilized de-identified data collected from existing records, ensuring no direct patient involvement or risk.
Author Contributions
Mohammad Abdulelah: data curation, conceptualization, and writing original draft and revision; Aleezay Asghar: conceptualization and writing original draft; Michael Sansait: writing-revision and project administration; Vida Rastegar: statistical analysis and writing-revision; Danielle Walsh: writing-original draft; Joshua Allgaier: project administration, writing-revision, and supervision; Nakul Ravikumar: conceptualization, project administration, supervision, and writing original draft and revision.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051-1059.
doi pubmed - Walsh M, Fritz S, Hake D, Son M, Greve S, Jbara M, Chitta S, et al. Targeted Thromboelastographic (TEG) blood component and pharmacologic hemostatic therapy in traumatic and acquired coagulopathy. Curr Drug Targets. 2016;17(8):954-970.
doi pubmed - Hartert H. [Blood clotting studies with Thrombus stressography; a new Investigation procedure]. Klin Wochenschr. 1948;26(37-38):577-583.
doi pubmed - Bugaev N, Como JJ, Golani G, Freeman JJ, Sawhney JS, Vatsaas CJ, Yorkgitis BK, et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: Practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;89(6):999-1017.
doi pubmed - Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: A systematic review and analysis. J Thromb Haemost. 2019;17(6):984-994.
doi pubmed - Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;2016(8):CD007871.
doi pubmed - Hartmann J, Dias JD, Pivalizza EG, Garcia-Tsao G. Thromboelastography-guided therapy enhances patient blood management in cirrhotic patients: a meta-analysis based on randomized controlled trials. Semin Thromb Hemost. 2023;49(2):162-172.
doi pubmed - Blatchford O, Davidson LA, Murray WR, Blatchford M, Pell J. Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ. 1997;315(7107):510-514.
doi pubmed - Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079.
doi pubmed - Vora P, Pietila A, Peltonen M, Brobert G, Salomaa V. Thirty-year incidence and mortality trends in upper and lower gastrointestinal bleeding in Finland. JAMA Netw Open. 2020;3(10):e2020172.
doi pubmed - Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633-1641.
doi pubmed - Hess JR, Ramos PJ, Sen NE, Cruz-Cody VG, Tuott EE, Louzon MJ, Bulger EM, et al. Quality management of a massive transfusion protocol. Transfusion. 2018;58(2):480-484.
doi pubmed - Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, Graupera I, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11-21.
doi pubmed - Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
doi pubmed - Sommer N, Schnuriger B, Candinas D, Haltmeier T. Massive transfusion protocols in nontrauma patients: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2019;86(3):493-504.
doi pubmed - Patil V, Shetmahajan M. Massive transfusion and massive transfusion protocol. Indian J Anaesth. 2014;58(5):590-595.
doi pubmed - Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482.
doi pubmed - American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122(2):241-275.
doi pubmed - Donovan K, Stanworth S, Jairath V. The optimal use of blood components in the management of gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42-43:101600.
doi pubmed - Razzaghi A, Barkun AN. Platelet transfusion threshold in patients with upper gastrointestinal bleeding: a systematic review. J Clin Gastroenterol. 2012;46(6):482-486.
doi pubmed - Lin VS, Sun E, Yau S, Abeyakoon C, Seamer G, Bhopal S, Tucker H, et al. Definitions of massive transfusion in adults with critical bleeding: a systematic review. Crit Care. 2023;27(1):265.
doi pubmed - Case JJ, Khan N, Delrahim M, Dizdarevic J, Nichols DJ, Schreiber MA, Deloughery TG, et al. Association of massive transfusion for resuscitation in gastrointestinal bleeding with transfusion-related acute lung injury. Indian J Crit Care Med. 2017;21(8):506-513.
doi pubmed - Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, Saluja V, et al. Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology. 2020;71(1):235-246.
doi pubmed - Lecleire S, Di Fiore F, Merle V, Herve S, Duhamel C, Rudelli A, Nousbaum JB, et al. Acute upper gastrointestinal bleeding in patients with liver cirrhosis and in noncirrhotic patients: epidemiology and predictive factors of mortality in a prospective multicenter population-based study. J Clin Gastroenterol. 2005;39(4):321-327.
doi pubmed - Orman ES, Roberts A, Ghabril M, Nephew L, Desai AP, Patidar K, Chalasani N. Trends in characteristics, mortality, and other outcomes of patients with newly diagnosed cirrhosis. JAMA Netw Open. 2019;2(6):e196412.
doi pubmed - Rizvi G, Marcinkowski B, Srinivasa N, Jett A, Benjenk I, Davison D, Yamane D. Impact on blood product utilization with thromboelastography guided resuscitation for gastrointestinal hemorrhage. J Intensive Care Med. 2023;38(4):368-374.
doi pubmed - Saeveraas SB, Seghatchian J, Sivertsen J, Hervig T. The use of thromboelastography (TEG) in massively bleeding patients at Haukeland University Hospital 2008-15. Transfus Apher Sci. 2019;58(1):117-121.
doi pubmed - Bhattacharyya A, Tewari P, Gupta D. Comparison of thromboelastography with routine laboratory coagulation parameters to assess the hemostatic profile and prognosticate postoperative critically ill patients. Ann Card Anaesth. 2021;24(1):12-16.
doi pubmed - Mutschler M, Paffrath T, Wolfl C, Probst C, Nienaber U, Schipper IB, Bouillon B, et al. The ATLS((R)) classification of hypovolaemic shock: a well established teaching tool on the edge? Injury. 2014;45(Suppl 3):S35-38.
doi pubmed - Lau JYW, Yu Y, Tang RSY, Chan HCH, Yip HC, Chan SM, Luk SWY, et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020;382(14):1299-1308.
doi pubmed - Gupta B, Garg N, Ramachandran R. Vasopressors: do they have any role in hemorrhagic shock? J Anaesthesiol Clin Pharmacol. 2017;33(1):3-8.
doi pubmed - Siddique SM, Mehta SJ, Lewis JD, Neuman MD, Werner RM. Rates of hospital readmission among medicare beneficiaries with gastrointestinal bleeding vary based on etiology and comorbidities. Clin Gastroenterol Hepatol. 2019;17(1):90-97.e93.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.