| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 2, April 2025, pages 56-62
Procalcitonin as a Predictor of Mortality in Patients With Severe Acute Pancreatitis
Luis Ricardo Ramirez-Gonzaleza, Leonardo Rafael Ordonez-Forestierya, Andrea Garciab, Maximiliano Cesar Iniguez-Martin-del-Campob, Francia Damary Llamas-Hernandezb, Kathia Dayana Morfin-Mezab, Samantha Emily Gonzalez-Munozb, Carlos Enrique Capetillo-Texsonb, Jose Pablo Gomez-Sierrab, Luis Osvaldo Suarez-Carreona, c, Gabino Cervantes-Guevarad, Enrique Cervantes-Pereze, Sol Ramirez-Ochoae, Andrea Socorro Alvarez-Villasenorf, Ana Olivia Cortes-Floresg, Alejandro Gonzalez-Ojedah, Clotilde Fuentes-Orozcob, i
aDepartment of General Surgery, Specialty Hospital, Western National Medical Center, Mexican Institute of Social Security, Guadalajara, Jalisco, Mexico
bBiomedical Research Unit 02, Specialty Hospital, Western National Medical Center, Mexican Institute of Social Security, Guadalajara, Jalisco, Mexico
cDepartment Surgical Clinics, Universidad de Guadalajara, Guadalajara, Jalisco, Mexico
dDepartment of Welfare and Sustainable Development, University Center of the North, University of Guadalajara, 46200, Colotlan, Jalisco, Mexico
eDepartment of Internal Medicine, Hospital Civil de Guadalajara Fray Antonio Alcalde, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara, Guadalajara 44280, Jalisco, Mexico
fDepartment of Medical Education and Health Research, Mexican Institute of Social Security, La Paz, Baja California Sur, Mexico
gDepartment of Oncological Surgery, ONKIMIA, Guadalajara, Jalisco, Mexico
hFaculty of Medicine of the University of Colima, Colima, Mexico
iCorresponding Author: Corresponding Author: Clotilde Fuentes-Orozco, Biomedical Research Unit 02, Mexican Institute of Social Security, Western National Medical Center, Specialty Hospital, Guadalajara, Jalisco, Mexico
Manuscript submitted February 18, 2025, accepted April 10, 2025, published online April 20, 2025
Short title: PCT as a Mortality Predictor in Severe AP
doi: https://doi.org/10.14740/gr2029
| Abstract | ▴Top |
Background: Acute pancreatitis (AP) is a severe inflammatory disorder that begins with the inappropriate activation of pancreatic enzymes within acinar cells due to biliary reflux, alcohol abuse, gallstones, and autoimmune disease. Several biomarkers have been studied that may aid in the early detection of pancreatic necrosis. The aim of this project was to evaluate the usefulness of procalcitonin (PCT) in predicting mortality in patients with severe AP in Mexican population.
Methods: An observational study, including 59 patients diagnosed with AP from 2018 to 2023, was conducted in a tertiary care hospital. Serum PCT levels were assessed on the first and third days of hospitalization (24 and 72 h).
Results: A total of 59 patients were included, and the main etiologies were lithiasis (28 patients, 47.5%) and endoscopic retrograde cholangiopancreatography (ERCP) (nine patients, 15.3%). Of the total patients, 16 (27.1%) died during their hospital stay, and the main etiologies were septic shock of abdominal origin (10 patients, 62.5%) followed by extra-abdominal shock (six patients, 37.5%). The average PCT level was 4.54 ± 8.12 on the first day of hospital stay, and 5.20 ± 10.90 at 72 h. The cut-off point was 1.26 ng/mL with the best sensitivity and specificity of PCT as a predictor of mortality at 72 h of 75% and 68%, respectively (area under the curve 0.7, 95% confidence interval (CI): 0.61 - 0.88), and positive and negative predictive values of 0.46 and 0.87, respectively.
Conclusions: We propose the usefulness of PCT as a biochemical marker to predict mortality in patients with severe AP due to its accessibility in the hospital environment. We propose to carry out studies with more patients and follow-up times. In addition, it is necessary to consider other biomarkers associated with PCT to help us improve the positive predictive value of mortality in this disease.
Keywords: Procalcitonin; Mortality; Severe acute pancreatitis
| Introduction | ▴Top |
Acute pancreatitis (AP) is a severe inflammatory process ranging from mild self-limiting involvement to death. It begins with the inappropriate activation of pancreatic enzymes within the acinar cells, triggered by various factors such as biliary reflux, alcohol abuse, gallstones, and autoimmune disease, which damages the acinar cells, causing their necrosis [1-3]. Additionally, AP is the most common and concerning complication after endoscopic retrograde cholangiopancreatography (ERCP), with incidence rates ranging from 1% to 40%. ERCP is widely recognized as an effective treatment for both benign and malignant conditions of the pancreaticobiliary system [4-6].
Pancreatitis should be suspected in patients with severe acute pain in the middle epigastrium or the left upper quadrant radiating to the back. However, diagnosis requires biochemical evidence (amylase or lipase levels three times above the standard limit) and radiological confirmation, typically through a computed tomography (CT) scan [7].
Based on the complications, AP can be classified as mild, moderately severe, or severe [8]. Most patients (80-85%) develop a mild, self-limiting course with a mortality rate of less than 1-3%. However, about 20% of patients develop moderate or severe AP, with a significantly higher mortality rate ranging from 13% to 35% [9]. This increased risk is primarily due to organ failure [10], usually in the early phase (in the first 3 days). The infection of the necrotic tissue, pancreatic or peripancreatic, is the leading cause of death in the late phase, with half of the deaths occurring in the first week of the disease [2, 8, 10].
Disease severity scoring systems and biomarkers have been used, either alone or in combination, to classify the severity of pancreatitis and predict outcomes [11]. Notable severity scoring systems include the Balthazar scale [12], the Bedside Index for Severity in Acute Pancreatitis (BISAP) [13], and the Acute Physiology and Chronic Health Evaluation (APACHE) II [14]. Biomarkers such as C-reactive protein, serum procalcitonin (PCT), and serum lactate dehydrogenase can aid in the early diagnosis of pancreatic necrosis [7].
Calcitonin prohormone, or PCT, is a polypeptide produced by various body tissues in response to releasing endotoxins or mediators. Proinflammatory cytokines, including interleukin-1, interleukin-6, and elevated tumor necrosis factor, upregulate its production [15, 16]. A value higher than 0.05 ng/mL indicates a possible infection. It begins to rise in the first 4 to 12 h; its circulating levels are reduced daily by half once the infection is controlled by antibiotics or the immune system [16].
The study aimed to determine a PCT cut-off point that has adequate sensitivity and specificity to correlate with mortality in the Mexican population with severe AP.
| Materials and Methods | ▴Top |
This was a retrospective non-randomized study. A total of 59 patients were enrolled in this diagnostic test study. These patients were treated at the Department of General Surgery of a tertiary care hospital in Guadalajara, Mexico. Serum tests were obtained for all patients diagnosed with severe AP between January 2019 and May 2023. Patients over 18 years old, regardless of gender, with a diagnosis of severe AP according to laboratory and imaging criteria were included. Patients under 18 years old, with incomplete clinical history, diagnosed with mild or moderate AP, who died 24 h before the suspected diagnosis, and in whom PCT values had not been obtained, were excluded.
The results of imaging studies were obtained from ultrasound, contrast-enhanced CT, and, if performed, ERCP.
Suspicion was determined by elevated amylase three times higher than average, elevated lipase, and elevated PCT, which are the criteria for assessing the severity of pancreatitis.
The scales used to determine the severity and to predict mortality were as follows.
Balthazar is a CT-based approach to assessing AP severity based on the appearance of the pancreas, first introduced in 1994 [12].
BISAP was proposed to help the early recognition of patients with mortality risk. The 5-point scoring system comprises five variables: blood urea nitrogen level > 25 mg/dL, impaired mental status, development of systemic inflammatory response syndrome (SIRS), age > 60 years, and presence of pleural effusion [13].
APACHE II includes 12 physiological variables (temperature, mean arterial pressure, heart rate, respiratory rate, A-a PO2 (FIO2 < 50%), arterial pH or HCO3, serum sodium, potassium, creatinine, hematocrit, white blood cell count, and a chronic health evaluation and age adjustment score). Each variable is weighted from 0 to 4, and the total score range is from 0 to 71 points [14].
PCT laboratory values were obtained at 24 and 72 h of hospitalization. Blood samples were taken and centrifuged for 10 min at 3,000 revolutions/minute at a temperature of -4 °C. Serum was withdrawn and stored at -80 °C. Serum PCT concentration was measured using the chemiluminescent immunoassay (LUMItest). The reference cut-off point established for the method was more significant than 0.05 ng/mL.
Statistical analysis
Statistical analysis was performed using SPSS for Windows 26.0 (SPSS Inc., Chicago, IL, USA). Response variables were presented as raw numbers or in percentages - descriptive phase with raw numbers, proportions, measures of central tendency, and dispersion. The Chi-squared test was used to compare the qualitative data. Quantitative variables were expressed as means, standard deviations (SDs), medians, and interquartile ranges (IQRs). Mann-Whitney U test was used for independent samples. A Spearman’s correlation was used for mortality. For the analysis of diagnostic tests to determine sensitivity and specificity, we used receiver operating characteristics (ROC) curves, as well as calculations of positive predictive value and negative predictive value. Any value of P <0.05 was considered statistically significant.
Ethical considerations
The study adhered to the provisions of the Declaration of Helsinki and its amendments, the General Health Law, and the regulations of the host institution on human research. The Local Health Research and Ethics Committee approved the protocol with registration R-2021-1301-156.
| Results | ▴Top |
Of the 59 patients in this study, 34 (57.6%) were men and 25 (42.4%) were women, with a median age of 50.73 ± 15.62 years. The average length of hospital stay was 17.36 ± 15.85 days. Sixteen (27.1%) of the patients died during their hospital stay, and the most commonly reported etiologies of morality were septic shock of abdominal origin (10 patients, 62.5%), followed by extra-abdominal shock (six patients, 37.5%).
A statistically significant difference was not found between survivors and non-survivors regarding gender, etiology, or Balthazar scale. However, there was a statistical significance in terms of APACHE II and BISAP scale (P = 0.020, P < 0.001), as shown in Table 1.
 Click to view | Table 1. Patient Characteristics and Main Clinical Data |
PCT levels were significantly higher in non-survivors than in survivors. At 24 h, in the non-survivor group, the median (IQR) value was 3.12 (0.76 - 12.16); in the survivor group, it was 0.79 (0.25 - 1.79) with a significant P-value of 0.024. Similarly, the PCT levels at 72 h were significantly higher in the non-survivor group (P = 0.003) (Table 2). Furthermore, Spearman’s correlation was made to determine the level of PCT at 24 and 72 h with the mortality where a P-value with statistical significance of P = 0.006 and P = 0.005 was obtained, respectively (Table 3).
 Click to view | Table 2. PCT Levels in Survivor Versus Non-Survivor Group |
 Click to view | Table 3. Correlation of PCT With Mortality |
The ROC curve was used to determine the potential mortality prediction. In the scales used, BISAP and APACHE II were the two scales with a significant prediction of mortality (P = 0.001 and P = 0.018, respectively) (Fig. 1, Table 4).
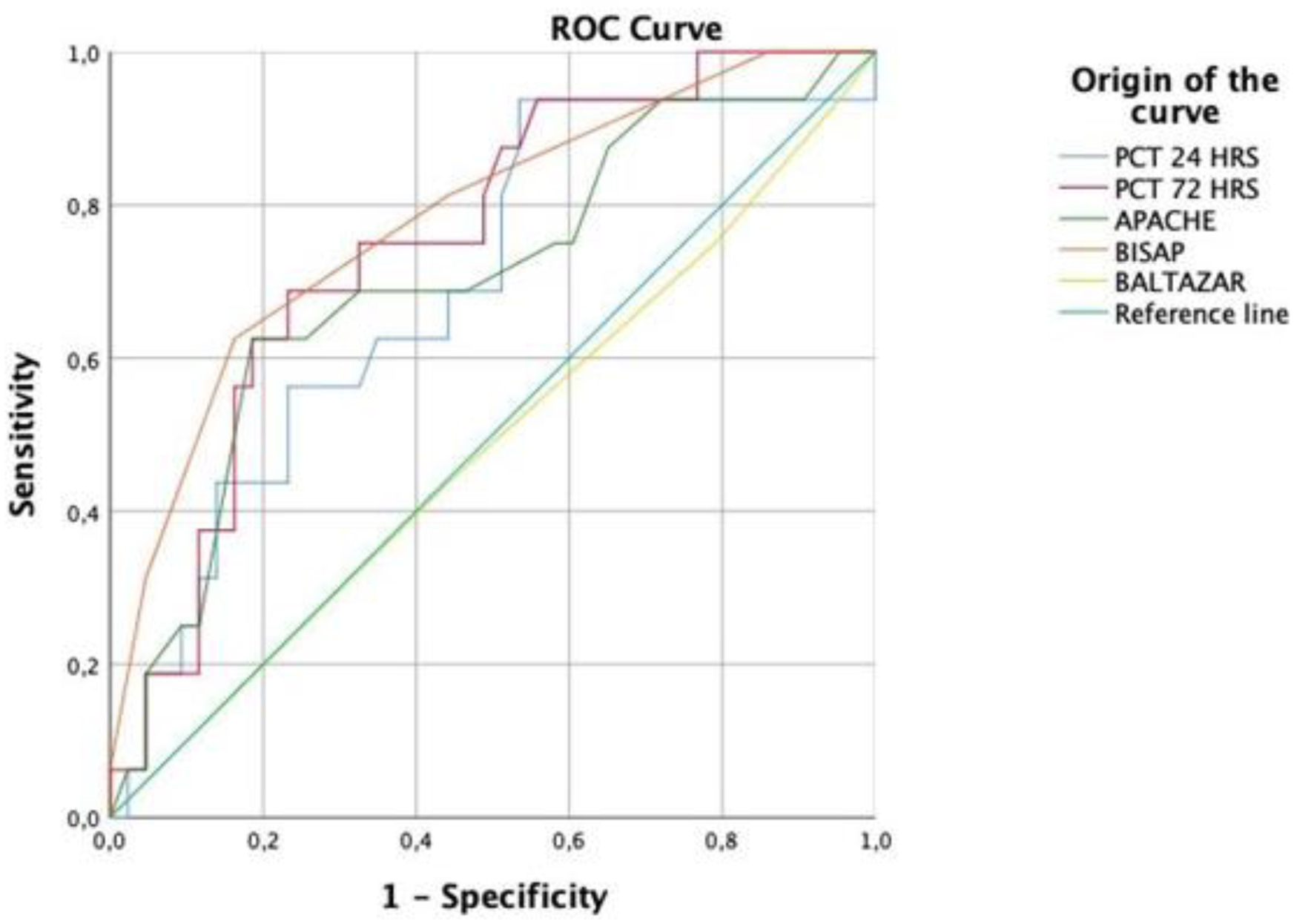 Click for large image | Figure 1. PCT and scales of ROC curve. PCT: procalcitonin; ROC: receiver operating characteristic. |
 Click to view | Table 4. APACHE II, BISAP and Balthazar Scales Analysis With Mortality |
The PCT level at 24 h with the best sensitivity and specificity had a cut-off point of 1.09 ng/mL. Moreover, at 72 h, a cut-off point of 1.26 ng/mL was obtained, with a sensitivity of 75% and a specificity of 68% (P = 0.003). The data are shown in Figure 1 and Table 5.
 Click to view | Table 5. Levels of PCT Compared With the Mortality at Different Times |
| Discussion | ▴Top |
AP has been a common abdominal disease, with increasing incidence and mortality rates over the past decade [17]. Therefore, early mortality prediction is a valuable tool for guiding effective treatment [18]. Our study used PCT to evaluate its effectiveness as a predictor of mortality in patients with severe AP, as it is an accessible, rapid, and widely available biomarker in our setting.
Infection is one of the most common complications in AP, often due to prolonged hospitalization and excessive anti-inflammatory therapy. A cohort study by Wu et al demonstrated that biliary origin is the most common etiology associated with mortality, mainly due to septic shock [17]; this aligns with our findings, which showed that 28 (47.5%) patients had lithiasis and septic shock of abdominal origin was the primary etiology of mortality, followed by extra-abdominal septic shock, observed in 10 (62.5%) and six (37.5%) patients, respectively.
In a study conducted by Jia et al [2] on the Chinese population, it was shown that PCT was the most successful test in terms of predicting severe AP in acute biliary pancreatitis, and they calculated an AUC of 0.84 for PCT. In addition, Kapiyamaz et al determined that the AUC for PCT in predicting mortality was 0.805 [19]. This is consistent with our data, where the AUC in predicting mortality at 24 h was 0.69, especially at 72 h with an AUC of 0.75.
Multiple studies explore the relationship between PCT levels and AP in conjunction with other scoring systems such as APACHE II, BISAP, and Balthazar [20-23]. Assessing PCT levels at different stages of hospitalization, precisely at 24 and 72 h, in combination with these scales, further supports the inclusion of these variables in our study, reinforcing the role of this biochemical marker in predicting mortality. APACHE II is the most widely used mortality predictor score for critically ill patients. Miko et al demonstrated in their meta-analysis that it is the most accurate scoring system for mortality despite its comprehensive range of items, which rely on thorough laboratory and imaging examinations. The BISAP score, on the other hand, predicts in-hospital mortality within the first 24 h of hospitalization [24].
Choudhuri et al in their study described that APACHE II scores and the serum PCT values at 48 h after admission were significantly higher in non-survivors compared to survivors (P < 0.001) [18]. As observed in our study, the PCT value after 72 h significantly predicts the mortality (P < 0.005). This finding can be related to its sensitivity (75%).
Pando et al analyzed the BISAP scale and mortality in patients with AP and found a sensitivity of 95.6% and a specificity of 58.1% [25]. Our study showed similar results, with a sensitivity of 81% and a specificity of 55%. Unlike the APACHE II and BISAP scales, the Balthazar score showed the lowest sensitivity at 43% and a specificity of 55%.
Samanta et al analyzed PCT values to predict poor outcomes in infected pancreatic necrosis, using a cut-off value of 1 ng/mL as a predictor. Our study found that a similar cut-off value of 1.09 ng/mL at 24 h and 1.26 ng/mL at 72 h had the best sensitivity and specificity, therefore showing similar results [26].
Comparing the PCT values at 24, the PCT value at 72 h is more significant in predicting mortality in AP; therefore, we recommended using the last value. In our study, PCT value of 4.54 ± 8.12 was recorded on the first day of hospital stay and a mean value of 5.22 ± 10.90 at 72 h, noticing an increase in the last value. Other studies have shown that increasing serum PCT levels over time are significantly associated with non-survivors (P < 0.001), indicating its capacity to predict mortality [18].
In addition, a significant difference was observed when PCT levels were compared at 24 and 72 h in survivors vs. non-survivors. Higher serum PCT levels were predominantly associated with increased mortality. This finding is corroborated by the retrospective cohort study conducted by Cavusoglu et al, which also demonstrated a significant difference in PCT levels between survivors and non-survivors (P < 0.001) [27].
Furthermore, Spearman’s correlation analysis revealed that elevated PCT levels at 72 h had a significantly positive correlation, indicating a strong association with mortality.
Conclusions
Our study shows the usefulness of PCT as a biochemical marker to predict mortality in patients with severe AP due to its accessibility in the hospital setting, as it is an available, fast, and valuable tool for early decision-making. We propose to carry out studies with more patients and follow-up times. In addition, it is necessary to consider other biomarkers associated with PCT that help us improve sensitivity and specificity as a predictor of mortality in our population.
Acknowledgments
We thank all the mothers who completed the questionnaire and all the people who helped us prepare the manuscript.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
The authors confirm that all patients provided their written consent for participation in the study.
Author Contributions
LRRG: conceptualization, formal analysis, methodology - original draft, and writing - review and editing. LROF: conceptualization, data curation, formal analysis, and writing - original draft. AG: conceptualization, data curation, formal analysis, and writing - original draft. MCIMC: conceptualization, investigation, visualization, and writing - original draft. FDLH: conceptualization, investigation, visualization, and writing - original draft. KDMM: writing - original draft and witing - review and editing. SEGM: writing - original draft and writing - review and editing. CECT: writing - original draft and writing - review and editing. JPGS: writing - original draft and writing - review and editing. LOSC: writing - original draft and writing - review and editing. GCG: writing - original draft and writing - review and editing. ECP: writing - original draft and writing - review and editing. SRO: writing - original draft and writing - review and editing. ASAV: writing - original draft and writing - review and editing. AOCF: writing - original draft and writing - review and editing. AGO: conceptualization, formal analysis, methodology,- original draft, and writing - review and editing. CFO: conceptualization, formal analysis, methodology, supervision, and writing - review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Severino A, Varca S, Airola C, Mezza T, Gasbarrini A, Franceschi F, Candelli M, et al. Antibiotic utilization in acute pancreatitis: a narrative review. Antibiotics (Basel). 2023;12(7):1120.
doi pubmed pmc - Jia Z, Xu J, Gu Y, Zheng L, Xia T. Values of different biochemical indices and clinical scoring systems for the assessment of acute biliary pancreatitis in a Chinese population. Am J Transl Res. 2023;15(5):3300-3308.
pubmed pmc - Li W, Ou L, Fu Y, Chen Y, Yin Q, Song H. Risk factors for concomitant infectious pancreatic necrosis in patients with severe acute pancreatitis: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2022;46(5):101901.
doi pubmed - Andrade-Davila VF, Chavez-Tostado M, Davalos-Cobian C, Garcia-Correa J, Montano-Loza A, Fuentes-Orozco C, Macias-Amezcua MD, et al. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85.
doi pubmed pmc - Fuentes-Orozco C, Davalos-Cobian C, Garcia-Correa J, Ambriz-Gonzalez G, Macias-Amezcua MD, Garcia-Renteria J, Rendon-Felix J, et al. Antioxidant drugs to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis: what does evidence suggest? World J Gastroenterol. 2015;21(21):6745-6753.
doi pubmed pmc - Martinez-Torres H, Rodriguez-Lomeli X, Davalos-Cobian C, Garcia-Correa J, Maldonado-Martinez JM, Medrano-Munoz F, Fuentes-Orozco C, et al. Oral allopurinol to prevent hyperamylasemia and acute pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2009;15(13):1600-1606.
doi pubmed pmc - Asim Riaz HM, Islam Z, Rasheed L, Sarfraz Z, Sarfraz A, Robles-Velasco K, Sarfraz M, et al. The evaluation of inflammatory biomarkers in predicting progression of acute pancreatitis to pancreatic necrosis: a diagnostic test accuracy review. Healthcare (Basel). 2022;11(1):27.
doi pubmed pmc - Yan X, Li J, Wu D. The role of short-chain fatty acids in acute pancreatitis. Molecules. 2023;28(13):4985.
doi pubmed pmc - Kong D, Lei Z, Wang Z, Yu M, Li J, Chai W, Zhao X. A novel HCP (heparin-binding protein-C reactive protein-procalcitonin) inflammatory composite model can predict severe acute pancreatitis. Sci Rep. 2023;13(1):9440.
doi pubmed pmc - Jiang WZ, Zhao HJ, Chen L, Tang XD, Deng Z. Clinical evaluation of continuous renal replacement therapy combined with peritoneal lavage for severe acute pancreatitis: a retrospective cohort study. Med Sci Monit. 2023;29:e939314.
doi pubmed pmc - Kandasamy S. Is it all clear if procalcitonin clears in acute pancreatitis? Indian J Crit Care Med. 2020;24(3):149-150.
doi pubmed pmc - Cheng T, Han TY, Liu BF, Pan P, Lai Q, Yu H, Cao Y. Use of modified Balthazar grades for the early prediction of acute pancreatitis severity in the emergency department. Int J Gen Med. 2022;15:1111-1119.
doi pubmed pmc - Gao W, Yang HX, Ma CE. The value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0130412.
doi pubmed pmc - Maves RC, Enwezor CH. Uses of procalcitonin as a biomarker in critical care medicine. Infect Dis Clin North Am. 2022;36(4):897-909.
doi pubmed - Mendez Hernandez R, Ramasco Rueda F. Biomarkers as prognostic predictors and therapeutic guide in critically ill patients: clinical evidence. J Pers Med. 2023;13(2):333.
doi pubmed pmc - Tian Y, Yao Y, Zhou J, Diao X, Chen H, Cai K, Ma X, et al. Dynamic APACHE II score to predict the outcome of intensive care unit patients. Front Med (Lausanne). 2021;8:744907.
doi pubmed pmc - Wu D, Ding J, Jia Y, Liu H, Xiao J, Peng J. Predictors of mortality in acute pancreatitis complicated with multidrug-resistant Klebsiella pneumoniae infection. BMC Infect Dis. 2021;21(1):977.
doi pubmed pmc - Choudhuri AH, Duggal S, Biswas PS, Uppal R. A comparison of acute physiology and chronic health evaluation II score and serum procalcitonin change for predicting mortality in acute pancreatitis. Indian J Crit Care Med. 2020;24(3):190-194.
doi pubmed pmc - Kayipmaz AE, Gedikaslan S, Aydogan RF. The laboratory parameters and scoring systems used to predict clinical outcomes in geriatric patients with acute pancreatitis. Eur Rev Med Pharmacol Sci. 2023;27(22):10899-10908.
doi pubmed - Shuanglian Y, Huiling Z, Xunting L, Yifang D, Yufen L, Shanshan X, Lijuan S, et al. Establishment and validation of early prediction model for hypertriglyceridemic severe acute pancreatitis. Lipids Health Dis. 2023;22(1):218.
doi pubmed pmc - Bao Y, Ge W. Correlation between serum levels of PTX-3, SIL-2R, inflammatory markers, and APACHE II scores in patients with severe acute pancreatitis. Medicine (Baltimore). 2022;101(43):e31252.
doi pubmed pmc - Gao N, Yan C, Zhang G. Changes of serum procalcitonin (PCT), C-reactive protein (CRP), interleukin-17 (IL-17), interleukin-6 (IL-6), high mobility group protein-B1 (HMGB1) and D-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (CRRT) and its clinical significance. Med Sci Monit. 2018;24:5881-5886.
doi pubmed pmc - Zhou J, Zhou P, Zhang Y, Wang G, Fan Z. Signal pathways and markers involved in acute lung injury induced by acute pancreatitis. Dis Markers. 2021;2021:9947047.
doi pubmed pmc - Miko A, Vigh E, Matrai P, Soos A, Garami A, Balasko M, Czako L, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis. Front Physiol. 2019;10:1002.
doi pubmed pmc - Pando E, Alberti P, Mata R, Gomez MJ, Vidal L, Cirera A, Dopazo C, et al. Early changes in blood urea nitrogen (BUN) can predict mortality in acute pancreatitis: comparative study between BISAP score, APACHE-II, and other laboratory markers-a prospective observational study. Can J Gastroenterol Hepatol. 2021;2021:6643595.
doi pubmed pmc - Samanta J, Dhar J, Birda CL, Gupta P, Yadav TD, Gupta V, Sinha SK, et al. Dynamics of serum procalcitonin can predict outcome in patients of infected pancreatic necrosis: a prospective analysis. Dig Dis Sci. 2023;68(5):2080-2089.
doi pubmed - Cavusoglu Turker B, Ahbab S, Turker F, Hoca E, Ciftci Ozturk E, Kula AC, Ozturk H, et al. Comparison of controlling nutritional status score with bedside index for severity in acute pancreatitis score and Atlanta classification for mortality in patients with acute pancreatitis. J Clin Med. 2024;13(12):3416.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.