| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 000, Number 000, June 2025, pages 000-000
Clinicopathological Features of KRAS-Mutated Colon Cancer: An Analytical Cross-Sectional Study
Edgar Joaquin Cortes-Torresa, Heli Hernandez-Gonzaleza, Kathia Dayana Morfin-Mezab, Andrea Garciab, Xochitl Monteon-Aspeitiab, Vianney Teresita Hernandez-Ramirezb, Carlos Enrique Capetillo-Texsonb, Jose Pablo Gomez-Sierrab, Alejandro Ruben Villasenor-Rodriguezb, Samantha Emily Gonzalez-Munozb, Sergio Jiram Vazquez-Sanchezb, Alejandro Gonzalez-Ojedac, Clotilde Fuentes-Orozcob, d
aInstituto Mexicano del Seguro Social (IMSS), Unidad Medica de Alta Especialidad (UMAE), Departamento de Cirugia General, Centro Medico Nacional de Occidente, Guadalajara, Jalisco, Mexico
bInstituto Mexicano del Seguro Social (IMSS), Unidad Medica de Alta Especialidad (UMAE), Unidad de Investigacion Biomedica 02, Centro Medico Nacional de Occidente, Guadalajara, Jalisco, Mexico
cFacultad de Medicina de la Universidad de Colima, Colima, Mexico
dCorresponding Author: Clotilde Fuentes-Orozco, Instituto Mexicano del Seguro Social (IMSS), Unidad Medica de Alta Especialidad (UMAE), Unidad de Investigacion Biomedica 02, Centro Medico Nacional de Occidente, Guadalajara, Jalisco, Mexico
Manuscript submitted February 26, 2025, accepted May 12, 2025, published online June 16, 2025
Short title: Features of KRAS-Mutated Colon Cancer
doi: https://doi.org/10.14740/gr2032
| Abstract | ▴Top |
Background: Colon cancer is a leading neoplasm worldwide, with 35% to 45% of colorectal cancer (CRC) patients exhibiting mutations in the Kirsten rat sarcoma oncogene (KRAS). This mutation affects disease development and serves as a biomarker for early detection, prognosis, and treatment. The objective of the present study was to identify the clinicopathological characteristics of colon cancer patients with KRAS mutations.
Methods: An analytical cross-sectional study involving patients with CRC was conducted. The study variables included sex, age, tumor location, KRAS and B-Raf proto-oncogene (BRAF) mutations, and the presence of metastases.
Results: The study involved 51 patients, with a mean (standard deviation) age of 61.4 ± 11.0 years. The most common tumor location was the sigmoid colon (35.3%), and 45.1% of patients were classified as tumor, node, metastasis (TNM) stage III with lymph node dissemination. Genetic analysis revealed that 35% of patients had KRAS mutations, while 32% had BRAF mutations. Notably, 61.1% of KRAS-positive patients also had BRAF mutations compared to 15.1% of KRAS-negative patients (P = 0.02).
Conclusions: KRAS-positive patients predominantly had tumors in the sigmoid colon. The coexistence of KRAS and BRAF mutations suggests a potential molecular interaction influencing disease progression. These findings highlight a distinct genomic pattern and the need for further research into its clinical implications.
Keywords: KRAS mutation; Colon cancer; Clinicopathological features; Staging
| Introduction | ▴Top |
Colorectal cancer (CRC) is one of the central neoplasms worldwide, becoming a focus of attention due to its high prevalence and mortality [1]. The World Health Organization has reported that it accounted for 10.4% of all cancer diagnoses in the last 5 years until 2020, with an age-adjusted incidence of 28.3 cases per 100,000 inhabitants annually [2]. This disease, which originates mainly in the epithelium of the colon and rectum, has shown an alarming increase in incidence, making it the second most diagnosed cancer globally [3].
The lifetime risk of CRC is similar in women and men, at 4.1% and 4.4%, respectively, although age-specific increases in incidence and mortality occur later in women than in men. The average age of diagnosis is 68 years in men and 72 years in women; however, in recent years, an increase in incidence has been observed in people younger than 50 years [4, 5].
About 80% of patients with colon cancer are sporadic; however, 20% have hereditary syndromes, which play a significant role in susceptibility to this disease. Syndromes such as Lynch syndrome or familial adenomatous polyposis follow an autosomal dominant inheritance pattern, where significant alterations occur in the genes associated with proteins responsible for repairing mismatch errors [2-4].
Certain diseases influence the site where this neoplasm appears; the behavior on the right side (cecum, ascending colon, hepatic angle, and transverse colon) is related to diseases not associated with polyps, such as Lynch syndrome and diabetes mellitus, since they share signaling pathway to cause damage to target organ [6]; on the left side (splenic angle, descending colon, and sigmoid colon), it is more frequent in patients with a history of polyp-related diseases [7].
CRC is a disease of significant genetic heterogeneity from different genetic and epigenetic pathways. The progression from adenoma to adenocarcinoma involves inactivation of tumor suppressor genes such as adenomatous polyposis coli (APC), tumor suppressor gene TP53 (TP53), and deleted in colorectal cancer (DCC) and mutations in Kirsten rat sarcoma oncogene (KRAS), suppressor of mothers against decapentaplegic (SMAD), and B-Raf proto-oncogene (BRAF), which lead to genomic instability. As CRC increases in size, the adenoma portion may disappear or remain part of the tumor [8].
Kirsten rat sarcoma is a commonly mutated oncogene in CRC in 40% of all cases; its mutations result in constitutive activation of the KRAS protein, which acts as a molecular switch to persistently stimulate downstream signaling pathways, including cell proliferation and survival, leading to tumorigenesis [9].
KRAS mutations are more associated with right colon cancer than left colon cancer; they are also less likely to have a first-degree relative with CRC or to be smokers [10].
Codons 12 (G12), 13 (G13), and 61 (Q61) are the most frequent sites by up to 85%; among them, codon 12 mutation is dominant and accounts for 65%; the remaining represent mutations affected at codons, 146, 51, and 117 low-grade tumors and have a less poor mismatch repair status [9].
The G12C mutation occurs at position 12 of the KRAS gene, where an amino acid cysteine (C) replaces glycine (G). This mutation produces a mutated KRAS protein with altered function, which remains constitutively active; it causes short responses to standard chemotherapy and worse overall survival than non-G12C mutations. Several KRAS G12C inhibitors have demonstrated clinical activity in recent years, although all patients eventually progressed [11].
Identifying negative feedback through the epidermal growth factor receptor (EGFR) has led to the development of KRAS inhibitors plus an anti-EGFR combination, which enhances antitumor activity [12].
Among the genetic changes found in CRC is the p. V600E pathogenic variant of the BRAF gene, which encodes a serine/threonine kinase involved in the EGFR-mitogen-activated protein kinase (MAPK) signaling pathway. This variant is vital to this cancer, as it causes constitutive activation of the protein, resulting in inhibition of apoptosis and uncontrolled cell proliferation [13].
EGFR is an important therapeutic target in CRCs. The essential nature of KRAS mutation testing in clinical practice to predict resistance to EGFR inhibitors during the treatment of metastatic CRC offers a promising outlook in the battle against this disease.
Although the mutation of the KRAS gene is one of the most studied genetic alterations in CRC, several uncertainties persist, such as regional differences in the frequency of KRAS mutations, the influence of comorbidities on its clinical impact, and its coexistence with other mutations that may modify the response to targeted therapies and the overall prognosis of the patient. Furthermore, the role of KRAS in the early stages of CRC, its function in metastasis, and how it influences resistance to treatments such as EGFR inhibitors remain subjects of ongoing research. This study aimed to describe these uncertainties regarding the clinicopathological characteristics and potential prognostic implications of KRAS mutations in a regional cohort of colon cancer patients in western Mexico.
| Materials and Methods | ▴Top |
An analytical cross-sectional study, which involved 51 patients over 18 years of age with a diagnosis of colon cancer who underwent KRAS mutation testing, was conducted at the Hospital de Especialidades de Centro Medico Nacional de Occidente by the microsurgery service from January 2022 to January 2023. The key study variables were sex, age, tumor location, cell differentiation, staging, and KRAS and BRAF mutation.
The present study adhered to the ethical principles for research on human beings of the World Medical Assembly established in the Declaration of Helsinki of 1989 and to the General Health Law of the United Mexican States, which establishes in article 17, and to the regulations for health research in Mexico. The protocols for carrying out the project were approved by the local research and ethics committee of the Mexican Social Security Institute under registration number R-2023-1301-191.
KRAS detection
Representative areas of tissue with an adequate sample of neoplastic cells were selected. Histopathology confirmed the diagnosis of adenocarcinoma of the colon or rectum.
After tissue acquisition, we performed genomic DNA extraction with the highly precise commercial QIAamp DNA Mini Kit (QIAGEN). The qualitative detection of mutations in codons 12, 13, 61, 117, and 146 of the KRAS oncogene and codon 600 of the BRAF oncogene was carried out with the KRAS Mutation Analysis Kit and BRAF codon 600 Mutation Analysis Kit II (EntroGen) on the Cobas Z 480 kit (Roche), which has a reported detection limit of 1% mutant DNA on a 99% base of normal DNA (KRAS-BRAF).
Statistical analysis
The data collected from the anatomic pathology service were meticulously emptied into an Excel database and subsequently into the SPSS v26 statistical program for univariate descriptive and bivariate comparative analysis.
Our study findings, which we will discuss in detail, hold significant implications for the understanding and treatment of colon cancer with KRAS mutation. We will present the results of our univariate descriptive and bivariate comparative analysis, which were conducted using the SPSS v26 statistical program. Qualitative variables were expressed as frequency and percentages, while quantitative variables were expressed as mean ± standard deviation (SD). For the bivariate analysis, qualitative variables were compared using the Chi-square test, and quantitative variables were compared using the Student’s t-test for small samples. Differences were considered significant with a P-value < 0.05.
| Results | ▴Top |
General characteristics
Fifty-one patients with confirmed diagnoses of colon cancer were included, with a predominance of male sex in 31 (60.8%) and a mean age of 61 years (SD 11), hypertension in 13 (25.5%), diabetes in 11 (21.6%), cardiovascular in five (9.8%), prostate cancer in two (3.9%), thyroid cancer, bladder cancer, and chronic obstructive pulmonary disease (COPD) in one (2%).
Anatomical site
Regarding the location of the tumor, 18 (35.3%) of the patients presented a tumor mass identified in the sigmoid colon, 13 (25.5%) in the rectum, 11 (21.6%) in the ascending colon, seven (13.7%) in the descending colon, and two (3.9%) in the transverse colon.
Histological characteristics
As for the histological grade concerning the colon tumor, 37 (72.5%) of the lesions evaluated were moderately differentiated tumors, eight (15.7%) reported well-differentiated lesions, and only six (11.8%) were poorly differentiated. The most frequent histological type was tubular adenocarcinoma, present in 48 (94.1%) of the colon tumors identified in this population.
Staging
These lesions presented a staging by the tumor, node, metastasis (TNM) system, predominantly in stage III, accounting for 23 (45.1%) of all lesions observed. In comparison, stage IV was present in 18 (35.3%), stage II corresponded to seven (13.7%), and three (5.9%) presented a stage I. However, in the evaluation of tumor size for TNM stage determination, it was perceived that 23 (45.1%) presented T3 lesions, 10 (19.6%) T2 lesions, and seven (13.7%) T4 lesions. Invasion of lymph node tissue was observed in 19 (37.3%) of the patients presenting N1, 16 (33.3%) N2, and 14 (29.4%) N0. In this sample of patients, 34 (66.7%) presented distant metastases at the time of evaluation.
Identification of the KRAS gene
Of the 51 patients tested, genetic characteristics were found in 18 (35.7%) with KRAS mutation; among these, 10 mutations (55.6%) occurred in codon 12, five (27.8%) in codon 13, two (11%) in codon 61, and one (5.6%) in codon 146. No mutations were observed in codon 117. In contrast, 33 patients (64.7%) did not exhibit KRAS mutations. In contrast, BRAF gene mutations were detected in 16 patients (31.4%), while 35 patients (68.6%) tested negative for BRAF alterations.
Our analysis of patients with colon cancer and KRAS mutation revealed a significant finding: those with KRAS+ were typically over 60 years of age, a stark contrast to patients without this mutation. The risk of comorbidities was also notably higher in patients with the mutation, particularly for diabetes mellitus and other diseases such as prostate cancer, thyroid cancer, bladder cancer, and COPD. However, the most striking revelation was the prevalence of the mutation in the sigmoid colon, as detailed in Table 1, underscoring the importance of this location in the context of colon cancer research.
 Click to view | Table 1. Characteristics of Patients With Colon Cancer With KRAS Mutation |
The results of histological grade and TNM staging are shown in Tables 2 and 3.
 Click to view | Table 2. Histological Characteristics of Patients With Colon Cancer |
 Click to view | Table 3. Tumor Staging of Colon Cancer Patients in Relation to KRAS Mutation |
No lymph node invasion and distant metastasis were identified in patients with KRAS mutation, presenting a higher proportion of N0 stage (P = 0.001), in contrast to patients without mutation, in which lymph node invasion was observed with a predominance of N1 stage (P = 0.18) (Fig. 1).
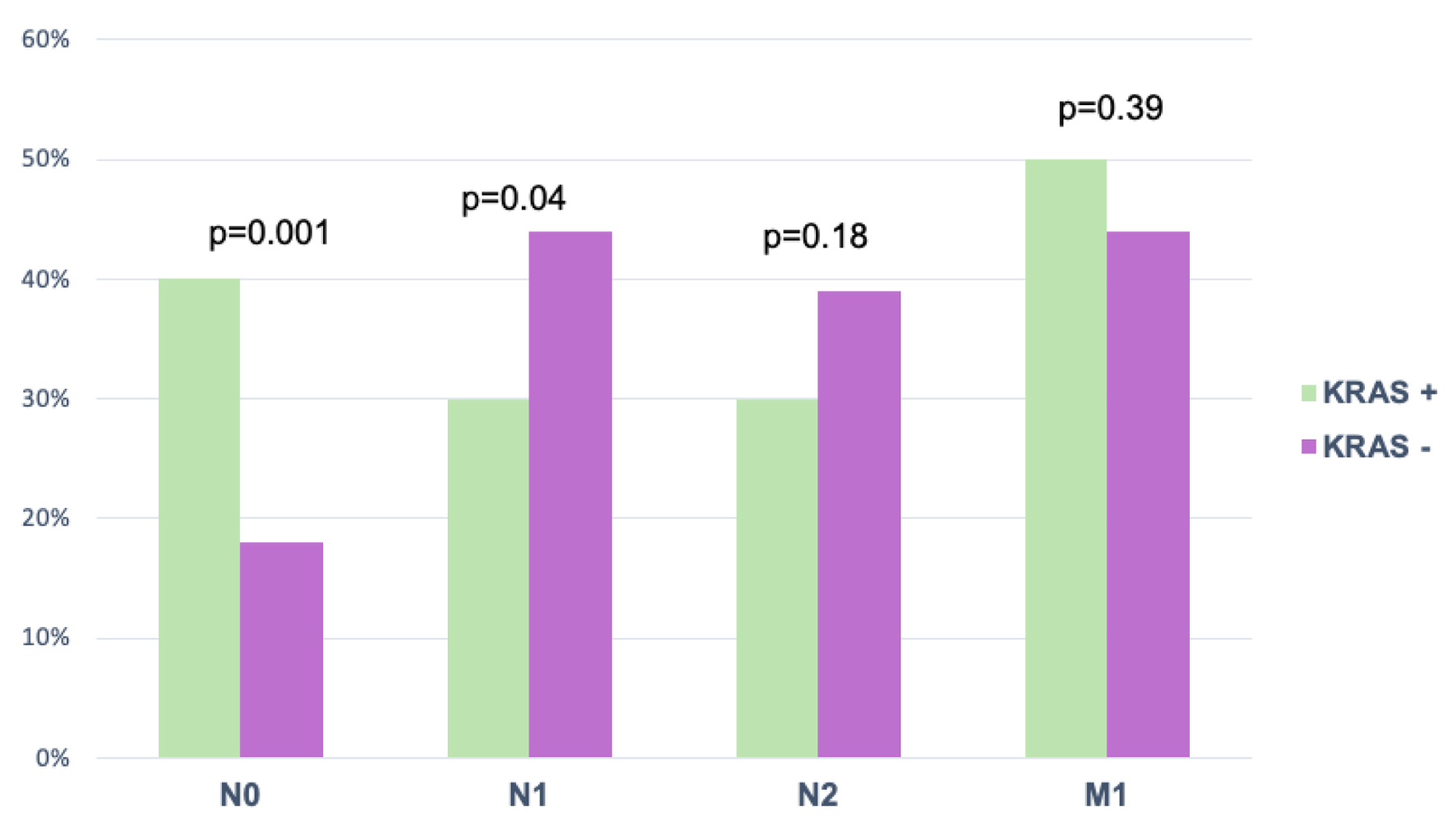 Click for large image | Figure 1. Nodal involvement and metastasis by TNM with KRAS. Values were expressed as percentages. Chi-square test was used for comparison. KRAS: Kirsten rat sarcoma oncogene; TNM: tumor, node, metastasis. |
All patients with KRAS mutations received standard chemotherapy; none received anti-EGFR, in accordance with clinical guidelines.
The patients with the presence of KRAS mutation and its association with BRAF mutation are shown in Table 4.
 Click to view | Table 4. KRAS and BRAF Mutation in Patients With Colon Cancer |
| Discussion | ▴Top |
The present study reveals a prevalence of 35.7% for KRAS and 32.1% for BRAF in our population. Similar data were mentioned by Lee et al [14] in a study conducted on the Korean population at Kangbuk Samsung Hospital in Seoul, South Korea. In this study, they evaluated 498 patients with colon cancer using KRAS sequencing. They identified a residual adenoma component, a precursor lesion of CRC [15], in 42 (8.4%) patients with CRC, which was associated with a high frequency of KRAS mutation (65%, P = 0.031). Among the 293 cases that received a KRAS mutation test, this mutation was identified in 37.9% of the population and located at codons 12 and 13 at a rate of 30.7% and 7.2%, respectively.
Yari et al [16] also supported these data in a study involving 100 consecutive patients with stage I to IV CRC who underwent surgical resection. They detected KRAS mutations in 29 patients (29%) at codons 12, 13, and 61 (72.4%, 20.7%, and 6.9%, respectively). However, unlike our findings, a study on the Mexican population conducted by Sanchez-Ibarra et al [17] in 2020 evaluated 500 patients with CRC from a hospital in northern Mexico and reported a KRAS mutation prevalence of 86%, with the most significant variant present in codon 12.
Patients with KRAS mutation were identified as older than 60 years in our population. They had a higher prevalence of comorbidities [18], specifically diabetes mellitus and other pathologies such as prostate, thyroid, bladder cancer, and COPD. Yari et al [16] reported similar findings, demonstrating that advanced age (> 60) was significantly associated with a higher rate of KRAS mutations. Regarding comorbidities, several studies have explored their association with KRAS, identifying an increased risk of mutation in the presence of metabolic, cardiovascular, and inflammatory diseases [19, 20]. These findings are consistent with our results and support the evidence linking KRAS mutations with advanced age and associated comorbidities.
The data showed that the sigmoid colon, as the anatomical site with the highest presence of KRAS mutation, is a crucial finding. Most cases did not show dissemination to lymph nodes, with stage N0 being more frequent in patients with the mutation and stage N1 in those without KRAS. These findings can guide treatment decisions and improve patient outcomes. However, this contrasts with Alharbi et al [21], who reported no significant differences between tumor location, clinical stage, or the presence of distant metastases. Kamphues [22], however, found that KRAS mutation in left-sided tumors decreases overall survival at 5 years due to the high risk of progression.
Study conclusions and contributions to disease understanding indicate that genetic variants vary substantially among different population groups. Therefore, it is relevant to contrast our data with that reported in the Mexican population by Sanchez-Ibarra et al [17]. Our study observed no differences between clinical stages, TNM categorization, or histological subtypes, contributing significantly to the understanding of the disease. However, we found greater involvement in the proximal colon for patients with KRAS mutation, whereas our study showed distal predominance in anatomical localization. The difference may be due to the sample size available in our study of patients with identified KRAS determination.
KRAS mutations were identified in 35.7% of patients, most frequently in codon 12 (55.6%), followed by codon 13 (27.8%), codon 61 (11%), and codon 146 (5.6%). These findings are consistent with those reported by Zhu et al who noted that mutations in codons 12 and 13 are the most common in CRC and are associated with resistance to anti-EGFR therapies and poor prognosis [9].
One of the most relevant findings in our population was identifying a significant association between KRAS and BRAF mutations, implying an interaction in the development and severity of the disease. Patients with positive KRAS reported a higher frequency of BRAF mutation [23] at 61.1%; in contrast, BRAF mutation was present in only 15.1% of patients without KRAS mutation. These data differ from Sanchez-Ibarra et al’s [17] research, which identified a 6% prevalence of BRAF mutation. Hernandez-Sandoval et al [13] reported a prevalence of around 4% in the western Mexican population with CRC. In contrast, the Latin American population presents a higher prevalence, reaching 15%, still below the rate reported in our study. Although KRAS and BRAF mutations have traditionally been considered mutually exclusive in the literature due to their redundant roles in the MAPK signaling pathway, recent studies like Woolley et al have challenged this paradigm. In an analysis of 6,605 tumor samples, they identified co-occurrence of mutations in specific molecular subgroups, finding that class 2 and class 3 BRAF mutations (non-V600E) frequently co-occur with atypical mutations in the Ras pathway (including KRAS) in up to 45.7% of cases with class 3 BRAF mutations [24]. Therefore, although uncommon, the high co-mutation rate observed in our cohort does not contradict current literature; rather, it aligns with recent findings highlighting the molecular complexity of BRAF mutations and their interaction with KRAS, particularly in advanced clinical settings or in populations with distinct genetic profiles.
The high prevalence of these variants in the present study suggests a possible interaction between oncogenes and a significant clinical role. However, it has been proposed that this interaction plays an essential role in treatment response [25]. A significant limitation of our study is the lack of long-term follow-up of patients, restricting our ability to understand the progression and prognosis of KRAS-mutated colon cancer fully. Long-term follow-up is crucial to assess survival, cancer recurrence, and response to different therapeutic modalities. Another significant area for improvement lies in the sample size; with only 51 patients, valuable and practice-relevant results can be obtained. However, due to the limited number of KRAS+ patients, the ability to generalize the findings to a broader population is limited.
Conclusion
In our study, we found that KRAS-positive patients predominantly presented with left-sided tumors, particularly in the sigmoid colon. The coexistence of KRAS and BRAF mutations suggests a potential molecular interaction that may influence disease progression. This finding could be attributed to a distinct genomic profile within the population studied, indicating a unique molecular pattern in CRCs. Further studies are warranted to elucidate the genetic interactions involved and their clinical implications.
Acknowledgments
We thank all the patients who agreed to participate in the study and all the people who helped us prepare the manuscript.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
The authors confirm that all patients provided their written consent for participation in the study.
Author Contributions
HHG: design of the work, analysis, wrote the main manuscript text; EJCT: design of the work, wrote the main manuscript text; KDMM: design of the work, analysis, interpretation of data, wrote the main manuscript text; AG: analysis, interpretation of data, wrote the main manuscript text; XMA: interpretation of data, wrote the main manuscript text; VTHR: interpretation of data; CECT: interpretation of data, wrote the main manuscript text; JPGS: interpretation of data; ARVR: interpretation of data; SJVS: analysis; SEGM: analysis; AGO: analysis, interpretation of data; CFO: design of the work, analysis, interpretation of data. All authors reviewed the manuscript.
Data Availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
Abbreviations
APC: adenomatous polyposis coli; BRAF: B-Raf proto-oncogene; COPD: chronic obstructive pulmonary disease; CRC: colon cancer; DCC: deleted in colorectal cancer; EGFR: epidermal growth factor receptor; KRAS: Kirsten rat sarcoma oncogene; QIAGEN: QIAamp DNA Mini Kit; SMAD: suppressor of mothers against decapentaplegic; TNM: tumor, node, metastasis; TP53: tumor suppressor gene TP53
| References | ▴Top |
- Sawicki T, Ruszkowska M, Danielewicz A, Niedzwiedzka E, Arlukowicz T, Przybylowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025.
doi pubmed - Organizacion Panamericana de la Salud. Organizacion Mundial de la Salud. Cancer today. 2020 [Internet]. Available from: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1.
- Afanador CH, Palacio KA, Isaza LF, Ahumada E, Ocampo CM, Muneton CM. Molecular characterization of colorectal cancer patients. Biomedica. 2022;42(Sp. 1):154-171.
doi pubmed - Hampel H, Kalady MF, Pearlman R, Stanich PP. Hereditary colorectal cancer. Hematol Oncol Clin North Am. 2022;36(3):429-447.
doi pubmed - Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164.
doi pubmed - Argiles G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291-1305.
doi pubmed - Flores-Altamirano M, Montiel-Jarquin AJ, Lopez-Colombo A, Lopez-Bernal CA, Garcia-Galicia A, Garza-Sanchez J. [Clinical and histopathological characteristics of malignant colon tumors by location]. Rev Med Inst Mex Seguro Soc. 2023;61(5):610-616.
doi pubmed - Mukund K, Syulyukina N, Ramamoorthy S, Subramaniam S. Right and left-sided colon cancers - specificity of molecular mechanisms in tumorigenesis and progression. BMC Cancer. 2020;20(1):317.
doi pubmed - Zhu G, Pei L, Xia H, Tang Q, Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20(1):143.
doi pubmed - Bonnot PE, Passot G. RAS mutation: site of disease and recurrence pattern in colorectal cancer. Chin Clin Oncol. 2019;8(5):55.
doi pubmed - Ros J, Vaghi C, Baraibar I, Saoudi Gonzalez N, Rodriguez-Castells M, Garcia A, Alcaraz A, et al. Targeting KRAS G12C mutation in colorectal cancer, a review: new arrows in the quiver. Int J Mol Sci. 2024;25(6):3304.
doi pubmed - Yoshino T, Van Cutsem E, Li J, Shen L, Kim TW, Sriuranpong V, Xuereb L, et al. Effect of KRAS codon 12 or 13 mutations on survival with trifluridine/tipiracil in pretreated metastatic colorectal cancer: a meta-analysis. ESMO Open. 2022;7(3):100511.
doi pubmed - Hernandez-Sandoval JA, Gutierrez-Angulo M, Magana-Torres MT, Alvizo-Rodriguez CR, Ramirez-Plascencia HHF, Flores-Lopez BA, Valenzuela-Perez JA, et al. Prevalence of the BRAF p.v600e variant in patients with colorectal cancer from Mexico and its estimated frequency in Latin American and Caribbean populations. J Investig Med. 2020;68(5):985-991.
doi pubmed - Lee HW, Song B, Kim K. Colorectal cancers with a residual adenoma component: Clinicopathologic features and KRAS mutation. PLoS One. 2022;17(9):e0273723.
doi pubmed - Torrecillas-Torres L, Cervantes-Sanchez G, Cardenas E, Martinez B, Reyes-Perez JA, Sanchez IC, et al. Recomendaciones para diagnostico y tratamiento del cancer de colon y recto en Mexico. Gaceta mexicana de oncologia. 2019;18(4):265-327.
- Yari A, Samoudi A, Afzali A, Karam ZM, Karimaldini NK, Abadi MFS, Ziasistani M, et al. Mutation status and prognostic value of KRAS and BRAF in southeast Iranian colorectal cancer patients: first report from southeast of Iran. J Gastrointest Cancer. 2021;52(2):557-568.
doi pubmed - Sanchez-Ibarra HE, Jiang X, Gallegos-Gonzalez EY, Cavazos-Gonzalez AC, Chen Y, Morcos F, Barrera-Saldana HA. KRAS, NRAS, and BRAF mutation prevalence, clinicopathological association, and their application in a predictive model in Mexican patients with metastatic colorectal cancer: A retrospective cohort study. PLoS One. 2020;15(7):e0235490.
doi pubmed - Quintana JM, Anton-Ladislao A, Lazaro S, Gonzalez N, Bare M, Fernandez-de-Larrea N, Redondo M, et al. Effect of comorbidities on long-term outcomes of colorectal cancer patients. Eur J Cancer Care (Engl). 2022;31(2):e13561.
doi pubmed - Brandstedt J, Wangefjord S, Nodin B, Eberhard J, Sundstrom M, Manjer J, Jirstrom K. Associations of anthropometric factors with KRAS and BRAF mutation status of primary colorectal cancer in men and women: a cohort study. PLoS One. 2014;9(2):e98964.
doi pubmed - Emilescu RA, Jinga M, Cotan HT, Popa AM, Orlov-Slavu CM, Olaru MC, Iaciu CI, et al. The role of KRAS mutation in colorectal cancer-associated thrombosis. Int J Mol Sci. 2023;24(23):16930.
doi pubmed - Alharbi A, Bin Dokhi H, Almuhaini G, Alomran F, Masuadi E, Alomran N. Prevalence of colorectal cancer biomarkers and their impact on clinical outcomes in Riyadh, Saudi Arabia. PLoS One. 2021;16(5):e0249590.
doi pubmed - Kamphues C, Kadowaki S, Amini N, van den Berg I, Wang J, Andreatos N, Sakamoto Y, et al. The interplay of KRAS mutational status with tumor laterality in non-metastatic colorectal cancer: An international, multi-institutional study in patients with known KRAS, BRAF, and MSI status. J Surg Oncol. 2021;123(4):1005-1014.
doi pubmed - Midthun L, Shaheen S, Deisch J, Senthil M, Tsai J, Hsueh CT. Concomitant KRAS and BRAF mutations in colorectal cancer. J Gastrointest Oncol. 2019;10(3):577-581.
doi pubmed - Woolley CE, Domingo E, Fernandez-Tajes J, Pennel KAF, Roxburgh P, Edwards J, Richman SD, et al. Coevolution of atypical BRAF and KRAS mutations in colorectal tumorigenesis. Mol Cancer Res. 2025;23(4):300-312.
doi pubmed - Guo L, Wang Y, Yang W, Wang C, Guo T, Yang J, Shao Z, et al. Molecular profiling provides clinical insights into targeted and immunotherapies as well as colorectal cancer prognosis. Gastroenterology. 2023;165(2):414-428.e417.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.