| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 3, June 2025, pages 119-128
High Seroprevalence of Helicobacter pylori and CagA/VacA Virulence Factors in Northern Central America
Juan E. Corrala, b, k, Dalton A. Norwoodc, k, Christian S. Alvarezd, Do Han Kime, Eleazar E. Montalvan-Sanchezf, Alvaro Rivera-Andradeg, Manuel Ramirez-Zeag, Katherine A. McGlynnd, Tim Waterboerh, Ricardo L. Dominguezi, Douglas R. Morganj, l
aDivision of Gastroenterology and Hepatology, Presbyterian Healthcare Services, Albuquerque, NM, USA
bDivision of Gastroenterology and Hepatology, Prisma Health, Greenville, SC, USA
cDivision of General Internal Medicine and Population Science, Department of Medicine, The University of Alabama at Birmingham, Birmingham, AL, USA
dDivision of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA
eDepartment of Medicine, Mount Sinai Morningside and West, Icahn School of Medicine at Mount Sinai, New York, NY, USA
fDepartment of Medicine, Section of Digestive Diseases, Yale School of Medicine, New Haven, CT, USA
gINCAP Research Center for the Prevention of Chronic Diseases (CIIPEC), Institute of Nutrition of Central America and Panama (INCAP), Guatemala City, Guatemala
hDivision of Infections and Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
iDepartment of Medicine, Western Regional Hospital, Santa Rosa de Copan, Honduras
jDivision of Gastroenterology and Hepatology, Department of Medicine, The University of Alabama at Birmingham, Birmingham, AL, USA
kThese authors shared the first authorship.
lCorresponding Author: Douglas Morgan, Division of Gastroenterology and Hepatology, The University of Alabama at Birmingham, Birmingham, AL, USA
Manuscript submitted March 18, 2025, accepted May 15, 2025, published online June 4, 2025
Short title: H. pylori and CagA/VacA in Central America
doi: https://doi.org/10.14740/gr2036
| Abstract | ▴Top |
Background: Northern Central America is unique in the Western Hemisphere, with a high incidence of gastric cancer, low/middle-income country (LMIC) status, and a substantial emigration to the United States. The two primary Helicobacter pylori (H. pylori) virulence factors related to carcinogenesis are cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA). The prevalence of these factors may help delineate gastric cancer risk in the region. We aimed to characterize the H. pylori seroprevalence and virulence factors in two Central American Countries (Honduras and Guatemala).
Methods: Healthy volunteers from Western Honduras and Central-Western Guatemala were recruited and tested for antibodies against 13 H. pylori antigens using a novel multiplex serology assay. H. pylori seropositivity was defined as positivity for ≥ 4 antigens, and active infection was defined as positivity for a combination of 2/4 antigens: VacA, GroEl, HcpC, and HP1564, based upon the literature. Multivariate logistic regression models were used to estimate the odds ratios for the association between H. pylori and CagA positivity.
Results: A total of 1,143 healthy adults were tested using the H. pylori multiplex serology assay (444 in Guatemala and 699 in Honduras). Mean age was 54.2 ± 14.5 years, 46.2% were male, 60% were from rural settings, and 56% lived > 1,000 meters above sea level. H. pylori prevalence was 87%, and 83% with active infection. The CagA and VacA seropositivity rates were 82% and 75%, respectively. No significant differences were noted according to country, age group, sex, or rural/urban location. None of the socioeconomic variables were significantly associated with the presence of H. pylori or CagA.
Conclusions: A high prevalence of H. pylori, CagA, and VacA is observed in Honduras and Guatemala, with implications for Northern Central America and immigrants from the region. Innovative and resource-appropriate primary and secondary prevention programs are needed.
Keywords: Gastric cancer; Helicobacter pylori; CagA; VacA; Central America
| Introduction | ▴Top |
The infection-associated cancers, gastric and cervical cancers, are the leading cancers in Northern Central America. Northern Central America is unique in the Western Hemisphere with a high incidence and mortality of gastric cancer, low/middle-income country (LMIC) status, and a high US immigrant population [1-3]. Central America has high incidence rates, including Guatemala and Honduras, with an estimated 12.2 and 8.3 cases per 100,000 individuals, respectively, although data are limited given the lack of population-based cancer registries (PBCRs) in the region [4, 5]. Chronic infection with Helicobacter pylori (H. pylori), the strongest risk factor for gastric adenocarcinoma, has an estimated prevalence of 70% in the two nations [6, 7].
H. pylori strains are genetically diverse, and variations in bacterial virulence factors correlate with the development of gastric adenocarcinoma. Cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) are the principal virulence factors associated with mucosal inflammation and carcinogenesis in humans [8-11]. In addition to strain-specific factors, host characteristics such as age, gender, germline mutations (e.g., cytokine polymorphisms), environmental influences (e.g., tobacco, diet, wood cookstove use), and coinfection (e.g., Epstein-Barr virus (EBV)) further delineate risk [12-14].
The H. pylori CagA protein is translocated into host cells by the type IV Cag secretion system after bacterial attachment. CagA interacts with phosphatidylserine and plays an important role in H. pylori infection [8, 10]. Inside the host cell, CagA is phosphorylated and induces morphological changes in gastric cells. Although all H. pylori strains induce gastritis, strains containing CagA augment the risk of severe chronic gastritis, multifocal atrophic gastritis, gastric intestinal metaplasia, and non-cardia gastric adenocarcinoma [8, 11, 15]. Approximately 85-100% of patients with duodenal ulcers have CagA+ strains, compared to 30-60% of infected patients who do not develop ulcers [16] and 63% of gastric cancer patients have CagA+ strains compared to 37.5% in controls [15]. The VacA toxin is a passive urea transporter that increases the permeability of the gastric epithelium to urea, thereby creating a favorable environment for H. pylori to live and reproduce. VacA toxin has also been shown to injure human gastric cells both in vitro and in vivo. CagA and VacA act synergistically; even though all H. pylori contain the gene coding for VacA, only those strains with the Cag pathogenicity island, including CagA, express the VacA toxin [11, 17]. Both CagA and VacA toxins are antigenic, can be measured in serum, and represent more specific biomarkers for gastric cancer prevention.
Few studies have described the prevalence of CagA and VacA virulence factors in the LMIC setting. Population studies describing the demographic and socioeconomic characteristics associated with virulent H. pylori strains are also scarce. This study evaluated the seropositivity of H. pylori in healthy individuals in Northern Central America and explored the demographic and socioeconomic characteristics of individuals harboring CagA/VacA-positive strains.
| Materials and Methods | ▴Top |
Study design and setting
This study used data from two population-based studies conducted in Guatemala (2016) and Honduras (2006 - 2012), two countries representative of the Central America Four (“CA-4”) region (Guatemala, Honduras, El Salvador, Nicaragua). The CA-4 is the largest LMIC region in the Western Hemisphere, with over 44 million inhabitants, which is linked to more than 5 - 8 million US immigrants [18]. The majority of the CA-4 population has Hispanic Mestizo ancestry 90-95%. In the areas of the study, Guatemala has a predominant Mayan-indigenous population in the Western highlands, and Honduras has a significant Maya-Cho’rti’ and Maya-Lenca population in the West and South regions [19]. The region has one of the highest gastric cancer incidence rates in the Western Hemisphere [20].
Healthy volunteers from Guatemala and Honduras were invited to participate. Research staff obtained informed consent from all the participants, administered the questionnaires, and performed a clinical evaluation. The questionnaire included questions on demographic variables, dietary habits, alcohol consumption, smoking, occupation, household characteristics, family history, and past medical history. Participants also underwent anthropometric measurements (height, weight, body mass index (BMI)), blood pressure assessment, and attainment of blood samples.
In Guatemala, participants were recruited in 2016 from five communities located in the Central and Western regions: Chichicastenango, Escuintla, Mixco, San Lucas Toliman, and San Pablo Jocopilas. In Honduras, participants were recruited from three departments in the Western region: Copan, Ocotepeque, and Lempira, between 2006 and 2012 [21]. Two locations in Guatemala (Escuintla and Mixco) were considered urban, and the remaining six locations were considered rural [22] (Fig. 1).
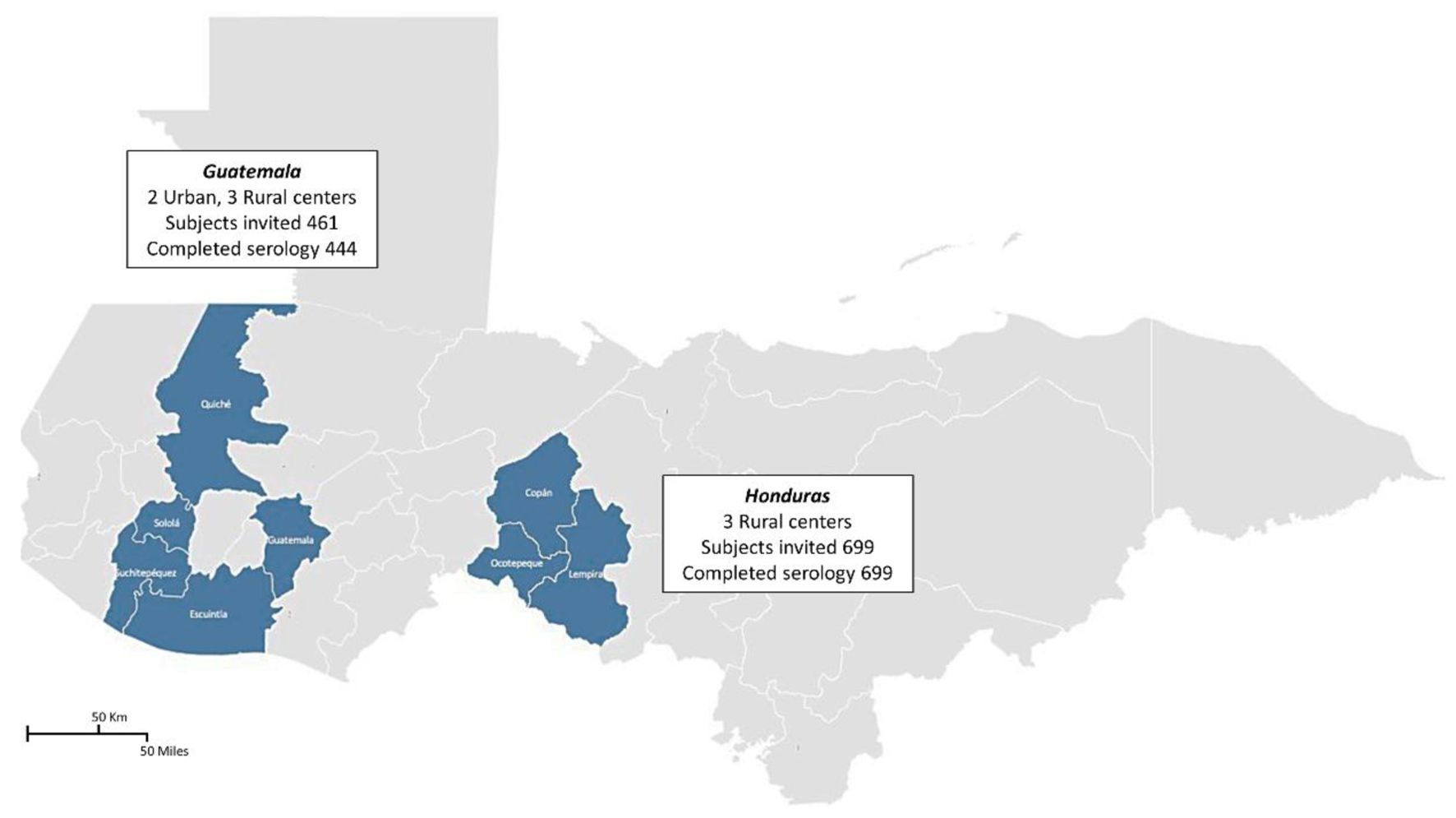 Click for large image | Figure 1. Sampling locations and participant recruitment across Northern Central America. This figure depicts the distribution and response rates of the subjects invited to participate in the study conducted in Northern Central America. In total, 1,143 participants were invited from various urban and rural centers within Guatemala and Honduras. The map highlights Guatemala with two urban centers and three rural centers, where 444 of the 461 subjects completed the serology tests. Similarly, in Honduras, all 699 invited subjects from three rural centers completed the tests. The shaded areas indicate the regions where the subjects were sampled, and the details provided within the insets specify the number of participants and completion rates of serological testing. |
Healthy volunteers were approached at the household level. Community maps were used to select households. In Guatemala, up to two non-genetically related persons per household were invited to participate in the study. In Honduras, every fifth household was chosen in two dozen villages in West-Central Honduras; the first appearing adult was invited to participate. The minimum ages of participation were 40 and 18 in Guatemala and Honduras, respectively. Exclusionary criteria included individuals with gastric cancer, peptic ulcer disease, prior abdominal surgery, and significant co-morbidities. Households were classified as urban or rural based on neighborhood characteristics. Alcohol consumption was defined as drinking ≥ 7 drinks per week in women and ≥ 14 drinks per week in men. Tobacco use was defined as individuals that have smoked at least 100 cigarettes or cigars in their lifetime. Antibiotic exposure was defined as the use of any antibiotics for any reason in the last 6 months. Additional details of the sampling methodology have been provided in previous publications [21, 23].
Laboratory testing
H. pylori seropositivity was determined using multiplex serology performed at the German Cancer Research Center in Heidelberg, Germany. The H. pylori multiplex serology assay measures antibodies against 13 H. pylori antigens (GroEl, UreA, HP0231, HP0305, NapA, HpaA, CagA, VacA, HcpC, HP1564, Catalase, Cad, and HyuA) measured by glutathione-S-transferase (GST)-tag fusion protein, which was performed based on published methodology [24, 25]. H. pylori positivity was defined as positivity for ≥ 4 antigens, and active infection was defined as the presence of two out of the four antigens: VacA, GroEl, HcpC, and HP1564 [24, 26]. Individuals who were seropositive for H. pylori were subdivided into those who expressed CagA antigens (CagA+) and those without CagA antigens (CagA-).
Statistical analysis
Descriptive statistics were calculated for each country and overall. The BMI values had a skewed distribution and were reported as median values with interquartile ranges (IQRs). Age was analyzed both as a continuous variable and as a categorical variable by decade. The primary analysis compared three groups based on multiplex serology: H. pylori-, H. pylori+ CagA-, and H. pylori+ CagA+. Analysis was performed with all participants who met the inclusion criteria, as well as for patients ≥ 40 years old (with exclusion of Honduras participants aged 18 - 39). The Pearson Chi-squared test and two-sample t-test were used to calculate differences in distributions between groups for categorical and continuous variables, respectively.
A secondary exploratory analysis was performed comparing three groups (H. pylori-, H. pylori+ CagA-, and all individuals CagA-) with individuals H. pylori+ CagA+, to identify any demographic and socioeconomic variables associated with CagA positivity. Univariate logistic regression was used to screen for any socioeconomic and clinical characteristics associated with CagA positivity, with a P-value < 0.1. Using only the variables considered to be associated with CagA positivity in the univariate analysis, multivariate models were computed to obtain adjusted odds ratios (ORs) for CagA positivity (the covariates included were age, sex, BMI, altitude, smoking, access to a refrigerator, and access to an electric or gas stove).
STATA software (STATA Corp, College Station, TX, USA) was used for statistical analysis. All tests for significance were two-sided. This study was conducted and reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational research. All the participants provided informed consent. This study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol and questionnaires were approved by the respective IRBs: Guatemala (#053-2015 Institute of Nutrition of Central America and Panama in Guatemala City, and #6877 Johns Hopkins Bloomberg School of Public Health in Baltimore, MD), Honduras (#IRB00011680 - IRB Western Hospital IRB FWA 00027925; UAB IRB 300003672), and analysis of de-identified information from both countries (exempt status 45 CFR 46.101(b)(4) Presbyterian Healthcare Services in Albuquerque, NM).
| Results | ▴Top |
Overall, 1,143 adults were enrolled in the studies and tested using the H. pylori multiplex assay (Guatemala, n = 444; Honduras, n = 699). The mean age was 54.2 ± 14.5 years and 46.2% were male. Nearly two-thirds of the participants (60%) lived in rural settings and 56% lived > 1,000 meters above mean sea level (mamsl). The most common occupations were domestic work (40%) and agriculture (19%). Approximately 53% (n = 608) of the individuals had a refrigerator, and 33.9% (n = 387) had an electric or gas stove. The percentage of underweight patients (BMI < 18.5) was 3.2%, while 23.5% were obese (BMI > 30). Twenty-seven percent (n = 310) were tobacco smokers and 43% (n = 488) consumed alcohol. The participants’ characteristics were similar in both countries, with only minor differences. Recent antibiotic use, access to a refrigerator, alcohol consumption, and tobacco smoking were more frequent in Guatemala (3.7, 3.4, 3.0, and 2.7 times higher, respectively) compared to Honduras (Table 1).
 Click to view | Table 1. Socioeconomic, Clinical, and Related Characteristics of Study Participants |
H. pylori positive serostatus was 86.9% (n = 993), and active H. pylori infection was observed in 83.0% (n = 949) of participants. CagA and VacA seropositivities were 82.4% (n = 941) and 75.4% (n = 861), respectively. In these prevalence estimates, there were no significant differences between countries (Fig. 2).
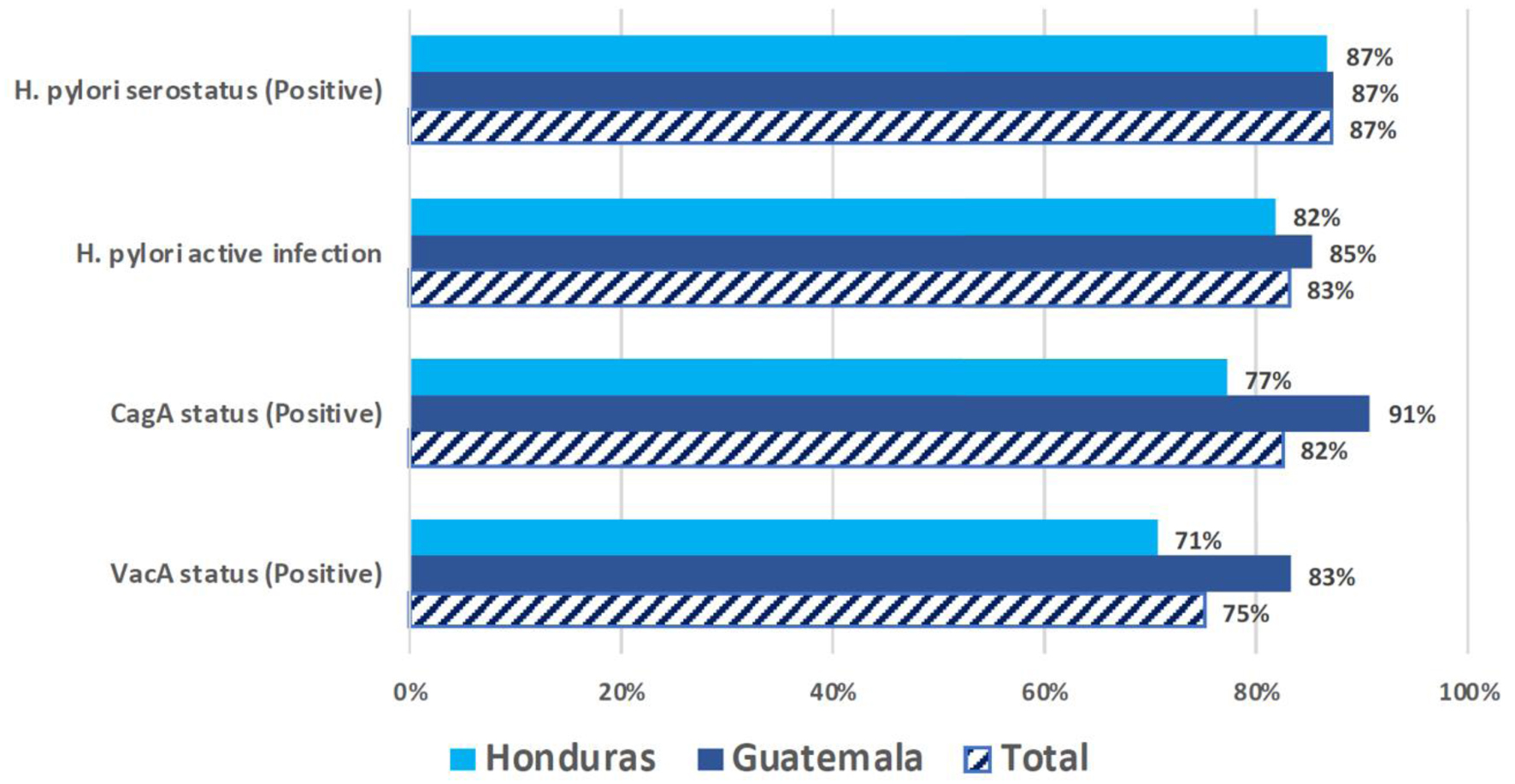 Click for large image | Figure 2. Prevalence of H. pylori, CagA, and VacA seropositivity by country (N = 1,143). This figure presents the prevalence rates of H. pylori serostatus and associated virulence factors among the populations sampled in Honduras and Guatemala. The bar chart compares the positive rates of H. pylori serostatus, active infection, and the presence of CagA and VacA virulence factors between the two countries as well as the total combined prevalence. |
The majority of participants who were H. pylori seropositive harbored a CagA+ strain (86.7%). No significant differences in H. pylori prevalence or CagA positivity were observed among the seven age groups (Fig. 3). Socioeconomic characteristics were similar across participants of the three serological groups (H. pylori-, H. pylori+ CagA-, and H. pylori+ CagA+), with several exceptions (Table 2). Smoking was more common among H. pylori CagA+ individuals. Participants with CagA+ H. pylori were less likely to have a refrigerator compared to H. pylori CagA- and seronegative participants. Individuals with H. pylori CagA+ were less likely to have an electric or gas stove than H. pylori seronegative participants. Analyses excluding Honduran participants 18 - 39 years old (N = 994, Supplementary Material 1, gr.elmerpub.com) showed only minor differences. Individuals with H. pylori+ CagA+ strains were significantly younger than the other two groups (mean age was 59.5, 60.6, and 57.0 years, respectively, in H. pylori- individuals, H. pylori+ CagA-, and H. pylori+ CagA+; P = 0.02). Antibiotic exposure was significantly lower in H. pylori+ CagA+ individuals (59.6% for H. pylori+, 63.8% for H. pylori+ CagA-, and 50.9% for H. pylori+ CagA+; P = 0.014).
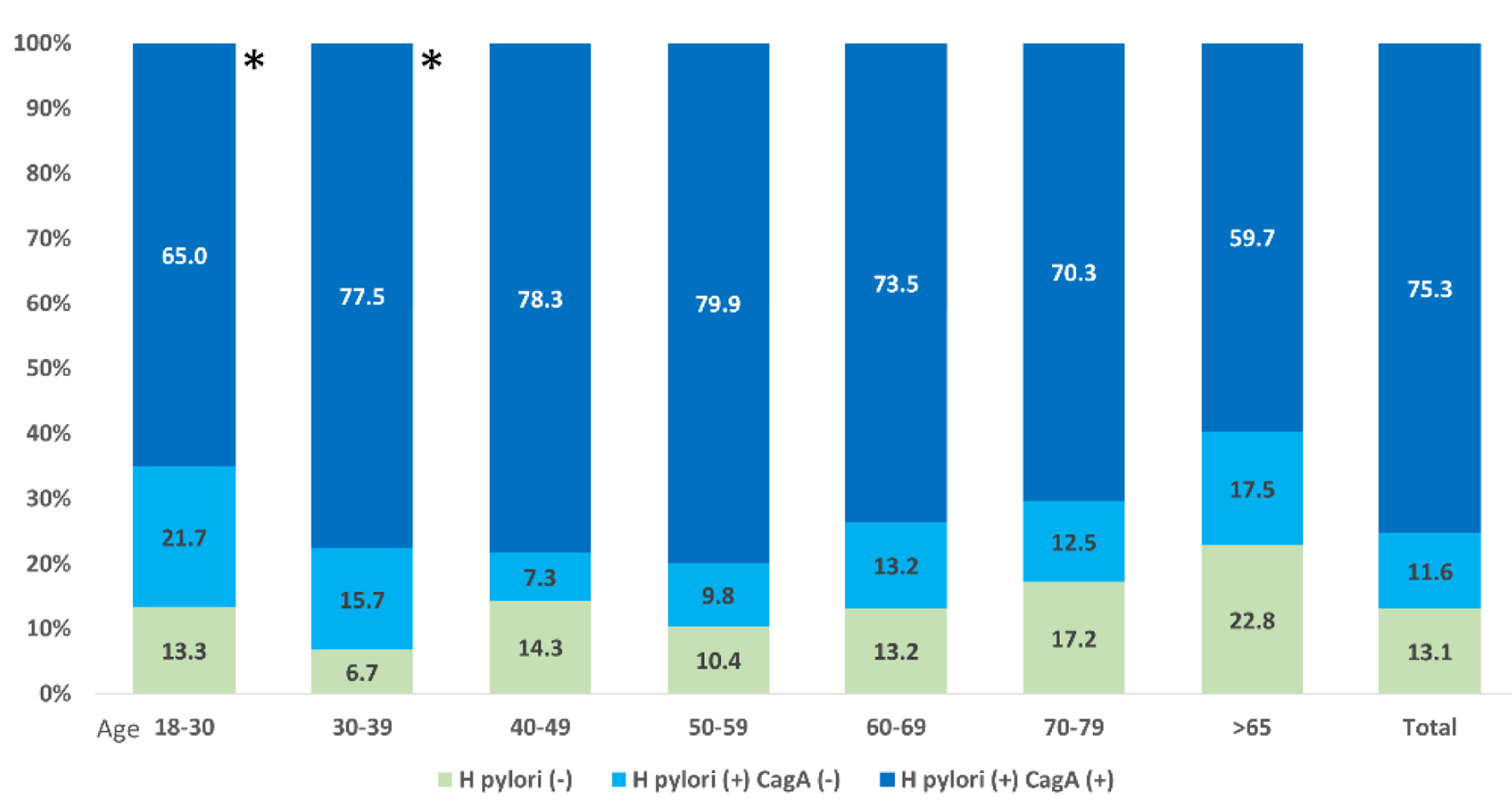 Click for large image | Figure 3. H. pylori and CagA seropositivity by age groups (N = 1,143). This figure illustrates the distribution of H. pylori and CagA seropositivity across different age groups among the 1,143 individuals sampled. The stacked bar chart shows the percentages of participants who were H. pylori-positive with and without CagA positivity (shown in darker and lighter shades of blue, respectively) and those who were H. pylori-negative (shown in green). Age groups were segmented as 18 - 30, 30 - 39, 40 - 49, 50 - 59, 60 - 69, and over 65 years (*Honduras participants only). |
 Click to view | Table 2. Socioeconomic and Clinical Characteristics Associated With CagA Positivity (N = 1,143) |
In the unadjusted analyses, advanced age, low BMI, access to household appliances (refrigerator and gas/electric stoves) were inversely associated with CagA positivity (Table 3). Tobacco use was directly associated with CagA positivity. In multivariate analyses, both low BMI (< 18.5) and lack of a household refrigerator were significantly and independently associated with increased odds of H. pylori CagA+ status. Compared to individuals who were H. pylori-negative, underweight participants had a lower likelihood of being CagA+ (adjusted OR (aOR) 0.37, 95% confidence interval (CI): 0.16 - 0.84, P = 0.018), as did those with both H. pylori and CagA negativity (aOR 0.47, 95% CI: 0.23 - 0.96, P = 0.039). Similarly, participants without a refrigerator had higher odds of CagA positivity when compared to H. pylori-negative individuals (aOR 0.56, 95% CI: 0.38 - 0.83, P = 0.004) and those negative for both H. pylori and CagA (aOR 0.67, 95% CI: 0.50 - 0.89, P = 0.006). Access to an electric or gas stove was inversely associated with CagA positivity (compared to H. pylori- individuals, aOR 0.65 (0.45 - 0.95)). Smoking was positively associated with CagA positivity (compared to H. pylori+ CagA- individuals, aOR 1.44 (1.04 - 1.99)).
 Click to view | Table 3. Exploratory Univariate and Multivariate Analyses of Socioeconomic and Clinical Characteristics Associated With CagA Positivity (N = 1,143) |
| Discussion | ▴Top |
We observed an enduring high prevalence of H. pylori infection and CagA positive strains in the LMICs of Northern Central America. H. pylori prevalence was 87% and 83%, overall and with active infection, respectively. The CagA and VacA seropositivity rates were 82% and 75%, respectively. Our results suggest a higher H. pylori prevalence than in previous studies in Guatemala (66.8%, N = 6,733) [6] and Honduras (82.6%, N = 259) [7], as well as a higher CagA prevalence compared with a prior report from Guatemala (53%) [27].
Despite the clear association between CagA and VacA strains and human disease, only a small proportion of individuals colonized by CagA+ H. pylori develop gastric cancer, which underscores the importance of host and environmental factors in gastric carcinogenesis. We found no statistically significant differences in H. pylori infection according to sex, age, BMI, or rural vs. urban residency. In multivariate analyses, refrigerator ownership and underweight status were inversely associated with H. pylori CagA+ infection. Compared to H. pylori-negative individuals, the aORs were 0.56 (refrigerator) and 0.37 (underweight); compared to individuals negative for both H. pylori and CagA, the aORs were 0.67 and 0.47, respectively. No other socioeconomic or clinical characteristics were statistically significant. The current literature suggests that H. pylori is acquired via fecal-oral or oral-oral transmission during childhood [5, 28]. We had hypothesized that improved living conditions may lower H. pylori seropositivity with a smaller proportion of CagA+ strains in younger participants; however, no significant trends were observed among the age groups.
Guatemala and Honduras are primarily populated by Mayan-Mestizo populations, with modest with ethnic differences [19]. The population in western regions in Honduras includes populations of Maya-Cho’rti’ and Maya-Lenca groups. This ethnic composition is similar to that of the Guatemalan Western highlands, and yet the plethora of Mayan indigenous groups in the Highlands are well recognized. The diets in the rural areas are primarily based on maize and beans, and similar environmental exposures have been reported in rural areas (e.g., woodstove cooking) [29, 30]. We did not identify significant differences in H. pylori prevalence, active infection, CagA positivity, or VacA positivity between the two countries. Our findings suggest that the prevalence of H. pylori high-risk strains are common to the CA-4 region. We report a higher prevalence of H. pylori+ CagA+ strains among smokers and individuals without access to refrigerators.
In endemic H. pylori areas with a high incidence of gastric cancer, the confirmation of the high prevalence of high-risk strains may inform prevention strategies. Mass screening and eradication of H. pylori should be considered in populations at higher risk of gastric cancer per the Taipei Consensus [31]. Novel strategies would be needed given the lack of resources in the health sector in Northern Central America. Previous geospatial analyses in western Honduras have demonstrated the utility of geospatial analysis to identify hot spots of gastric cancer and high-risk strains [32].
To our knowledge, this is the first large community study to examine the prevalence of H. pylori and risk strains in Northern Central America. The age ranges for inclusion in Honduras and Guatemala were modestly different (> 18 and > 40 years old, respectively), but exclusion of the younger individuals in Honduras did not have a significant effect on the overall results. The novel and validated H. pylori multiplex assay was used. This multiplex serology assay has adequate sensitivity (85.7%, 95% CI: 68.5-94.3%) and negative predictive value (97.3%, 95% CI: 93.2-98.9%) when compared to the fecal antigen test [25], and has been validated against enzyme-linked immunosorbent assay, western blot, and urea breath tests [24, 26]. Serologic tests may be less sensitive both with decreased H. pylori burden (e.g., intestinal metaplasia, cancer) or with decreased immune response (e.g., age, advanced cancer), which were not significant factors in our study.
Despite adequate test performance, this assay has limitations inherent to antigen testing. Antigen expression can change during the natural history of H. pylori infection. As humans develop atrophic gastritis and intestinal metaplasia, the H. pylori bacterial load decreases, lowering CagA exposure. However, CagA can still be detected in patients with advanced disease, as observed in patients with gastric adenocarcinoma after tumor resection.
Conclusion
H. pylori infection with high-risk strains remains endemic in the Northern Central America, and supports the reality of the high incidence of gastric cancer in the area. This has implications for cancer control in this LMIC region, wherein the infection-associated cancers, gastric and cervical, continue to define a large proportion of the cancer burden. There are additional implications for Mesoamerica and immigrants to the USA from this region. Novel approaches are imperative in light of the resource limitations in the Ministries of Health.
| Supplementary Material | ▴Top |
Suppl 1. Socioeconomic and clinical characteristics associated with CagA positivity, individuals 40 years or older (N = 994).
Acknowledgments
We acknowledge the staff working on the regional research centers.
Financial Disclosure
US National Cancer Institute (NCI) (DRM, P01 CA028842, R03 CA167773, K07 CA125588) and the NCI Center for Global Health (DRM, P30CA068485, PAR-15-155).
Conflict of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Author Contributions
CSA, DRM, and JEC involved in conceptualization. DAN, DHK, EEMS, ARA, MRZ, and RLD involved in data acquisition. CSA, KAM, TW, and DRM involved in methodology. DAN, CSA, DRM, and JEC involved in data analysis/interpretation. DAN, CSM, DRM, and JEC wrote the original draft. CSA, ARA, MRZ, KAM, RLD, and TW critically reviewed and edited the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
BMI: body mass index; CagA: cytotoxin-associated gene A; H. pylori: Helicobacter pylori; IQR: interquartile range; LMICs: low/middle-income countries; mamsl: meters above mean sea level; SD: standard deviation; VacA: vacuolating cytotoxin A
| References | ▴Top |
- Ruiz de Campos L, Valdez de Cuellar M, Norwood DA, Carrasco TY, Montalvan-Sanchez EE, Rodriguez Funes MV, Beasley T, et al. High incidence of gastric cancer in El Salvador: a national multisectorial study during 2000 to 2014. Cancer Epidemiol Biomarkers Prev. 2024;33(12):1571-1577.
doi pubmed - Pena-Galo EM, Palacios-Navarro G, Pastora-Membreno J, Torres-Herman T, Norwood DA, Montalvan-Sanchez EE, Beasley T, et al. High gastric cancer mortality and years of life lost in Nicaragua: a population-based study 1997 to 2012. Cancer Epidemiol Biomarkers Prev. 2024;33(12):1564-1570.
doi pubmed - Dominguez RL, Montalvan-Sanchez EE, Norwood DA, Rodriguez-Murillo A, Dominguez L, Estevez Ordonez D, Beasley T, et al. Population-based study of gastric cancer survival and associations in rural Western Honduras. Cancer Epidemiol Biomarkers Prev. 2024;33(12):1578-1585.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29(3):559-578.
doi pubmed - Diaz Y, de Leon J, Rivera L, Matta V. Prevalencia de la infeccion por Helicobacter pylori en la poblacion que asistio a las clinicas de APROFAM durante 2006-2011. Ciencia, Tecnologia y Salud. 2017;4(2):217-226.
- Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, Cook P, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2013;24(2):209-215.
doi pubmed - Blaser MJ. Role of vacA and the cagA locus of Helicobacter pylori in human disease. Aliment Pharmacol Ther. 1996;10(Suppl 1):73-77.
doi pubmed - Figura N. Helicobacter pylori exotoxins and gastroduodenal diseases associated with cytotoxic strain infection. Aliment Pharmacol Ther. 1996;10(Suppl 1):79-96.
doi pubmed - Spechler SJ, Fischbach L, Feldman M. Clinical aspects of genetic variability in Helicobacter pylori. JAMA. 2000;283(10):1264-1266.
doi pubmed - Wroblewski LE, Peek RM, Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713-739.
doi pubmed - Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, et al. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24(2):249-256.
doi pubmed - Morgan DR, Dominguez RL, Keku TO, Heidt PE, Martin CF, Galanko JA, Omofoye OA, et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol. 2006;4(9):1103-1111.
doi pubmed - Norwood DA, Montalvan-Sanchez E, Dominguez RL, Morgan DR. Gastric cancer: emerging trends in prevention, diagnosis, and treatment. Gastroenterol Clin North Am. 2022;51(3):501-518.
doi pubmed - Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125(6):1636-1644.
doi pubmed - Weel JF, van der Hulst RW, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat GN, et al. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996;173(5):1171-1175.
doi pubmed - Tummuru MK, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18(5):867-876.
doi pubmed - Pineros M, Frech S, Frazier L, Laversanne M, Barnoya J, Garrido C, Gharzouzi E, et al. Advancing reliable data for cancer control in the central America four region. J Glob Oncol. 2018;4:1-11.
doi pubmed - Demographic Diversity and Change in the Central American Isthmus. Santa Monica, CA: RAND Corporation, 1997.
- Norwood DA, Montalvan-Sanchez EE, Corral JE, Estevez-Ordonez D, Paredes AA, Dominguez LB, Rodriguez AA, et al. Western Honduras Copan population-based cancer registry: initial estimates and a model for rural central America. JCO Glob Oncol. 2021;7:1694-1702.
doi pubmed - Alvarez CS, Florio AA, Butt J, Rivera-Andrade A, Kroker-Lobos MF, Waterboer T, Camargo MC, et al. Associations between Helicobacter pylori with nonalcoholic fatty liver disease and other metabolic conditions in Guatemala. Helicobacter. 2020;25(6):e12756.
doi pubmed - Rivera-Andrade A, Kroker-Lobos MF, Lazo M, Freedman ND, Smith JW, Torres O, McGlynn KA, et al. High prevalence of non-alcoholic fatty liver disease and metabolic risk factors in Guatemala: A population-based study. Nutr Metab Cardiovasc Dis. 2019;29(2):191-200.
doi pubmed - Smith JW, Kroker-Lobos MF, Lazo M, Rivera-Andrade A, Egner PA, Wedemeyer H, Torres O, et al. Aflatoxin and viral hepatitis exposures in Guatemala: Molecular biomarkers reveal a unique profile of risk factors in a region of high liver cancer incidence. PLoS One. 2017;12(12):e0189255.
doi pubmed - Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter. 2009;14(6):525-535.
doi pubmed - Butt J, Varga MG, Blot WJ, Teras L, Visvanathan K, Le Marchand L, Haiman C, et al. Serologic response to helicobacter pylori proteins associated with risk of colorectal cancer among diverse populations in the United States. Gastroenterology. 2019;156(1):175-186.e172.
doi pubmed - Butt J, Blot WJ, Shrubsole MJ, Varga MG, Hendrix LH, Crankshaw S, Waterboer T, et al. Performance of multiplex serology in discriminating active vs past Helicobacter pylori infection in a primarily African American population in the southeastern United States. Helicobacter. 2020;25(1):e12671.
doi pubmed - Fernandez-Botran R, Wellmann IA, Une C, Mendez-Chacon E, Hernandez de Rodas E, Bhandari B, Villagran de Tercero CI. Seroprevalence of helicobacter pylori/CagA antibodies in Guatemalan gastric cancer patients: association of seropositivity with increased plasma levels of pepsinogens but not soluble urokinase plasminogen activator receptor. Am J Trop Med Hyg. 2020;103(1):260-265.
doi pubmed - Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640.
doi pubmed - Pena AS, Crusius JB. Central America in transition: from maize to wheat challenges and opportunities. Nutrients. 2015;7(9):7163-7171.
doi pubmed - Rifkin SB, Miller AK, Montalvan-Sanchez EE, Norwood DA, Martinez E, Waterboer T, Beasley TM, et al. Wood cookstove use is associated with gastric cancer in Central America and mediated by host genetics. Sci Rep. 2023;13(1):16515.
doi pubmed - Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69(12):2093-2112.
doi pubmed - Dominguez RL, Cherry CB, Estevez-Ordonez D, Mera R, Escamilla V, Pawlita M, Waterboer T, et al. Geospatial analyses identify regional hot spots of diffuse gastric cancer in rural Central America. BMC Cancer. 2019;19(1):545.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.