| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 000, Number 000, June 2025, pages 000-000
Efficacy and Safety of Mycophenolate Mofetil Compared to Azathioprine in Autoimmune Hepatitis: A Meta-Analysis
Shahryar Khana, g, Mashal Alam Khanb, Aamer Ahmadb, Hamza Asifc, Muhammad Safwanb, Asmad Khanb, Muhammad Waqar Elahid, Mihir Prakash Shahe, Yousaf Zafarf
aDepartment of Medicine, University of Kansas Medical Center, Kansas City, KS, USA
bKhyber Medical University, Peshawar, Pakistan
cDepartment of Medicine, University of Louisville, Louisville, KY, USA
dDepartment of Medicine, West Virginia University School of Medicine, Morgantown, WV, USA
eDepartment of Medicine, University of Oklahoma, Norman, OK, USA
fDepartment of Medicine, University of Mississippi Medical Center, Jackson, MS, USA
gCorresponding Author: Shahryar Khan, Department of Medicine, University of Kansas Medical Center, Kansas City, KS, USA
Manuscript submitted April 18, 2025, accepted May 30, 2025, published online June 16, 2025
Short title: MMF vs. AZA in Autoimmune Hepatitis
doi: https://doi.org/10.14740/gr2044
| Abstract | ▴Top |
Background: Mycophenolate mofetil (MMF) has been suggested as a potential alternative treatment option for patients who are intolerant or unresponsive to the standard corticosteroid and azathioprine (AZA) regimen for autoimmune hepatitis (AIH). This systematic review and meta-analysis aimed to comprehensively evaluate and compare the biochemical efficacy and safety profiles of MMF and AZA in the treatment of AIH.
Methods: This review systematically examined the available literature from the inception of the MEDLINE and EMBASE databases up to November 2024. The primary outcomes of interest included the evaluation of biochemical remission (BR), the effectiveness of MMF in patients who were non-responsive (AZA-NR) or intolerant to azathioprine (AZA-IT), and the assessment of adverse events (AEs) and overall survival.
Results: This meta-analysis evaluated 11 studies comprising 952 participants, with 57.45% receiving MMF and the remaining receiving AZA. The findings indicate that MMF demonstrated a significantly higher BR rate (88.57%) than AZA (53.64%). The pooled analysis revealed a substantial improvement in the BR rate with MMF compared to AZA (odds ratio (OR): 7.81, 95% confidence interval (CI): 2.21 - 27.69). Furthermore, the estimated enhancement in treatment efficacy with MMF was 61% (95% CI: 42.63 - 78.04) among AZA-NR patients and 61.73% (95% CI: 54.88 - 68.35) in AZA-IT patients. However, the analysis did not reveal any significant differences between the two groups in terms of AEs (OR: 0.57, P = 0.47) and overall survival (OR: 1.27, P = 0.64).
Conclusions: MMF may be a suitable first-line alternative to AZA for AIH, with higher rates of BR, especially in patients intolerant or non-responsive to standard therapy. However, the long-term efficacy and safety of MMF requires further investigation through rigorous randomized controlled trials.
Keywords: AIH; MMF; AZA; Treatment-naive; Intolerant; Non-responsive; Biochemical remission; Adverse events
| Introduction | ▴Top |
Autoimmune hepatitis (AIH) is a chronic inflammatory liver condition caused by the disruption of the immune regulatory system, leading to autoimmune activity and liver cell damage. This disorder is prevalent globally and affects individuals across diverse ages and ethnicities, with a predisposition towards females [1]. The clinical presentation of AIH demonstrates substantial variability, ranging from non-specific symptoms such as fatigue and upper abdominal discomfort to polymyalgia and joint arthralgia [2]. The diagnosis of this condition relies on a comprehensive evaluation of clinical, biochemical, and histological findings, with diagnostic scoring systems that further inform clinical decision-making. Key biochemical abnormalities include elevated serum transaminase levels and alkaline phosphatase levels and hypergammaglobulinemia. Ultimately, the diagnosis of AIH necessitates a thorough assessment of these factors, collectively contributing to a definitive diagnosis and guiding the implementation of appropriate long-term immunosuppressive management strategies for the affected population [3].
Untreated AIH can progress rapidly, with early research indicating mortality rates as high as 40% within 6 months for individuals with severe, untreated disease [4, 5]. The goal of AIH treatment is to induce and maintain complete suppression of inflammatory activity, thereby preventing progression to cirrhosis and liver decompensation [6]. Without treatment, reported 5- and 10-year survival rates are 50% and 10%, respectively [7]. The standard first-line intervention for AIH involves a combination of corticosteroids and the immunosuppressant drug azathioprine (AZA), which can lead to remission in 65-80% of patients [8]. However, a significant proportion, around 20% of patients, either do not respond adequately to or cannot tolerate this conventional corticosteroid-AZA therapy [9]. This underscores the critical need for alternative treatment approaches in patients who fail to achieve a satisfactory response or cannot tolerate the standard regimen.
Mycophenolate mofetil (MMF) has been proposed as a potential rescue therapy for individuals who are intolerant or unresponsive to the standard corticosteroid-AZA regimen [10]. However, the existing evidence supporting its use is primarily derived from small retrospective studies, which have limitations in providing a comprehensive evaluation of its efficacy and safety profiles. Additionally, there are currently no specific guideline recommendations regarding the application of MMF in the management of AIH [11]. This lack of robust, prospective data and clear guidance highlights the critical need for further research to evaluate the biochemical efficacy and safety of MMF with AZA for the treatment of this complex autoimmune liver condition. This comprehensive assessment aimed to provide valuable insights to guide clinicians in making informed decisions and in enhancing the overall management of patients with AIH. The primary objective of this study was to evaluate the efficacy of MMF as a first-line treatment compared to standard therapy. Additionally, this study sought to investigate the effectiveness of MMF in patients who were intolerant or unresponsive to the standard corticosteroid-AZA regimen.
| Materials and Methods | ▴Top |
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. Given the absence of patient-specific data, ethical approval was not required for this study.
Inclusion and exclusion criteria
The review included studies that met the following criteria: randomized controlled trials (RCTs), prospective or retrospective cohort studies, or case-control studies; studies comparing the use of MMF with standard therapy for the treatment of AIH in adult patients; and studies evaluating the efficacy of treating refractory AIH with corticosteroids and MMF in patients who failed to respond or were intolerant to standard treatment. Eligible studies reported relevant outcome measures, such as biochemical remission (BR) and safety profiles. Studies were excluded if they lacked a comparison group; were published in a language other than English; were case reports; editorials, or letters; or did not assess relevant outcome measures. This meta-analysis focused on evaluating the primary outcome of BR, defined as normalized transaminases and IgG levels with or without histological normalization, within the initial 2 years of treatment. Treatment failure was defined heterogeneously, although generally characterized by a composite of factors. The principal indicators of failure included an inadequate BR, evidenced by a failure to normalize liver enzyme and IgG levels, and the persistence of histological activity on liver biopsy despite therapeutic intervention. Supplementary Material 1 (gr.elmerpub.com) presents a comparative summary of the diverse definitions of BR and treatment failure used in the studies analyzed. Despite these variations, most studies generally adhered to the 2010 American Association for the Study of Liver Diseases (AASLD) guidelines for defining a BR. Other outcomes assessed were adverse events (AEs) and overall survival. To further investigate the efficacy of MMF as a second-line therapy, a subgroup analysis was performed to compare its effects in patients who were non-responsive and those intolerant to standard therapy. This allowed for an assessment of the differential impact of MMF in these two distinct patient populations, who utilized the drug as a rescue option.
Search strategy and study selection
We designed a comprehensive search strategy and implemented it across EMBASE and PubMed, covering all publications from inception to November 15, 2024. This strategy combines free text and MeSH terms, incorporating synonyms and spelling variations. The full search strategy is available in Supplementary Material 2 (gr.elmerpub.com). We also manually searched the reference lists of all identified trials, guidelines, and reviews on the topic. All citations were imported into Covidence. Two reviewers (AA and MK) independently screened the titles, abstracts, and full-text articles, and discrepancies were resolved by a third reviewer (SK). Data extraction was performed in duplicate by AA and MK using standardized forms, including study identification (e.g., authorship, publication year, country of origin), study design and risk of bias assessment, patient demographics (e.g., age, sex, comorbidities), intervention and comparator descriptions, and outcomes. Relevant subgroup data where available were also collected.
Data synthesis and analysis
The analysis utilized odds ratios (ORs) with 95% confidence intervals (CIs) to evaluate categorical outcomes and mean differences (MDs) with 95% CIs for continuous outcomes. Statistical significance was defined as an alpha criterion ≤ 0.05. A random-effects modeling approach was employed to estimate the pooled effects, and forest plots were used to visually present the meta-analysis results. Additionally, a random-effects model with restricted maximum likelihood estimation was used to address the observed heterogeneity across the included studies. Furthermore, Wald-type CIs were calculated based on the pooled effect size and its associated standard error to quantify the uncertainty surrounding the summary statistics. The degree of heterogeneity was evaluated using I2 statistics, which provide a quantitative measure of inconsistency across study results. The I2 values were interpreted as follows: 0-30% indicated low heterogeneity, 30-60% moderate heterogeneity, 50-90% substantial heterogeneity, and 75-100% considerable heterogeneity, with a P-value < 0.1 considered statistically significant recommended by the Cochrane Handbook. Due to the inclusion of fewer than 10 studies in the final analysis, a formal evaluation of publication bias was not performed. Sensitivity analyses were conducted by removing each study individually and including only those studies without a high risk of bias. We assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. Statistical analyses were conducted using RevMan version 5.4 and MedCalc version 19.4 software.
| Results | ▴Top |
The study selection process adhered to PRISMA guidelines, as outlined in Supplementary Material 3 (gr.elmerpub.com). A comprehensive literature search identified 495 and 486 citations from PubMed and Embase, respectively. After removing duplicates, 897 articles were screened, and 11 full-text studies with a total of 952 participants were ultimately included [13-23]. The study design was predominantly retrospective with two prospective studies. Eleven studies were conducted from 2008 to 2024 across 10 countries: Greece, the Netherlands, Belgium, the USA, Sweden, Germany, the United Kingdom, Switzerland, Portugal, and Australia. The baseline characteristics of the included studies are shown in Table 1 [13-23]. Approximately 57.45% of the participants were treated with MMF, whereas the remaining received AZA. The mean age of participants was 50.4 years in the MMF group and 49.3 years in the AZA group. Female participants constituted the majority of the study population, accounting for 78% of the MMF group and 73% of the AZA group. The MMF dose across studies ranged 1.5 - 2 g/day, whereas AZA doses were reported as 1 - 2 mg/kg/day. Baseline cirrhosis was reported in 27% and 23% of patients in the MMF and AZA groups, respectively. The overall certainty of the evidence was evaluated using the GRADE approach and is presented in Supplementary Material 4 (gr.elmerpub.com).
 Click to view | Table 1. Characteristics of Included Studies |
BR rate in MMF and AZA
The meta-analysis findings suggested that MMF was associated with a notably higher BR rate of 88.57% (95% CI: 76.84-96.85%), as opposed to 53.64% with AZA (95% CI: 33.5-73.19%). The pooled analysis showed a substantial improvement in the BR rate with MMF compared to AZA (OR: 7.81, 95% CI: 2.21 - 27.69, I2 = 77%, P = 0.001; Fig. 1). However, heterogeneity across the included studies was high, as reflected by an I2 value of 77%. With the exclusion of Hlivko et al due to a high risk of bias and Snijder et al given its RCT design, the I2 improved to 0. The results remained consistent, indicating that continued MMF demonstrated a statistically significantly improved BR (OR: 25.1, 95% CI: 12.5 - 50.4, I2 = 0%, P < 0.05). Sensitivity analysis focusing solely on treatment-naive AIH patients revealed that MMF exhibited consistent improvements in sustained BR compared with AZA (OR: 6.60, 95% CI: 1.61 - 27.09, I2 = 82%, P = 0.009). Furthermore, the BR at 6 months was not different between the two groups (OR: 3.08, 95% CI: 0.72 - 13.22, I2 = 73%, P = 0.13, Fig. 2), while one study reported a sustained BR at 12 months favoring MMF (OR: 2.47, 95% CI: 1.09 - 5.7, P = 0.03) [22].
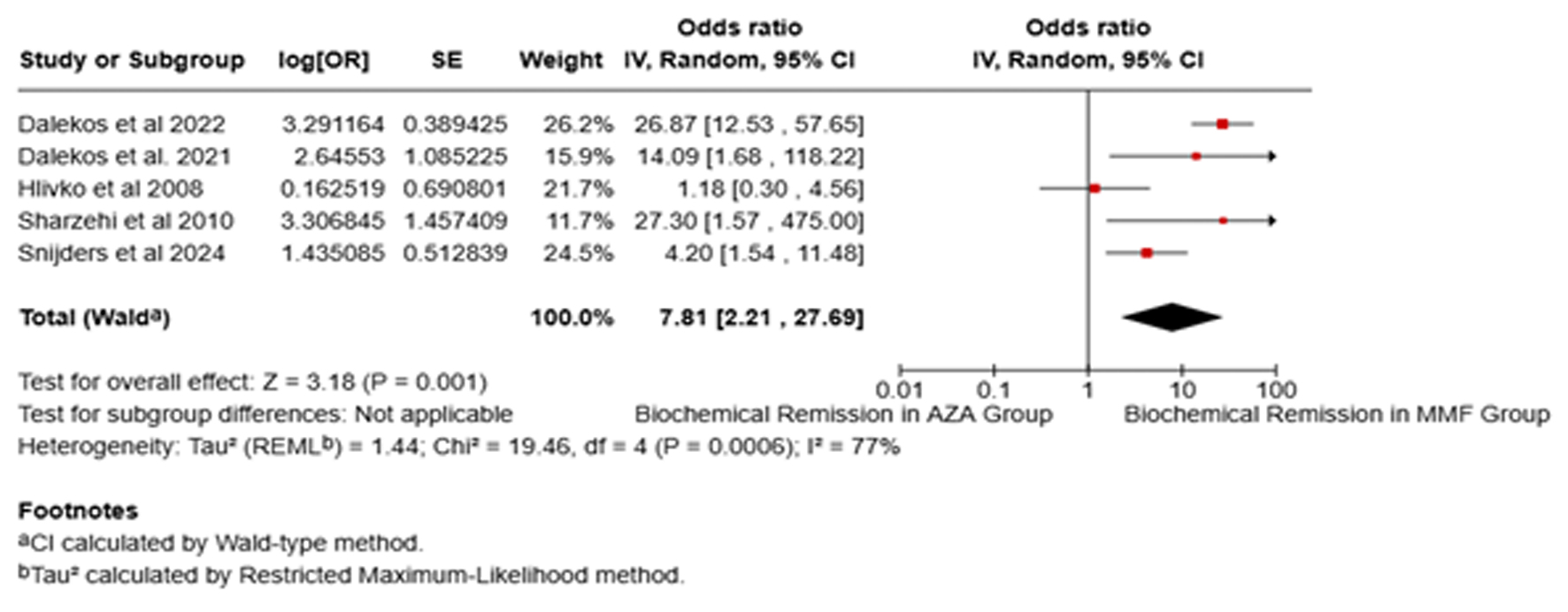 Click for large image | Figure 1. Forest plot of comparison of mycophenolate mofetil (MMF) with azathioprine (AZA) for biochemical remission. |
 Click for large image | Figure 2. Forest plot of comparison of mycophenolate mofetil (MMF) with azathioprine (AZA) for biochemical remission at 6 months. |
BR rate with MMF following AZA non-response and intolerance
The subgroup analysis of eight studies [13-20] examined the efficacy of MMF in patients with AIH who had previously failed or were intolerant to AZA therapy. The pooled results showed that MMF achieved a 61% (95% CI: 42.63-78.04%, I2 = 74%, Fig. 3) BR rate among AZA non-respondent (AZA-NR). When studies with a moderate to high risk of bias were excluded from the sensitivity analysis, the pooled results maintained consistency; however, a high degree of heterogeneity remained. Although this subgroup also experienced a 3.87% relapse rate and a 31.46% treatment failure rate with MMF. Similarly, in AZA intolerant (AZA-IT) participants, MMF demonstrated a comparable remission rate of 61.73% (95% CI: 54.88-68.35%, I2 = 1.9%, Fig. 4), with minimal heterogeneity across the studies, suggesting consistent findings. However, the pooled analysis for the AZA-IT subgroup revealed a 5.65% relapse rate and 17.43% treatment failure rate with MMF. These findings suggest that MMF may be an effective alternative treatment option for individuals with AIH who are unable to tolerate or respond adequately to AZA therapy.
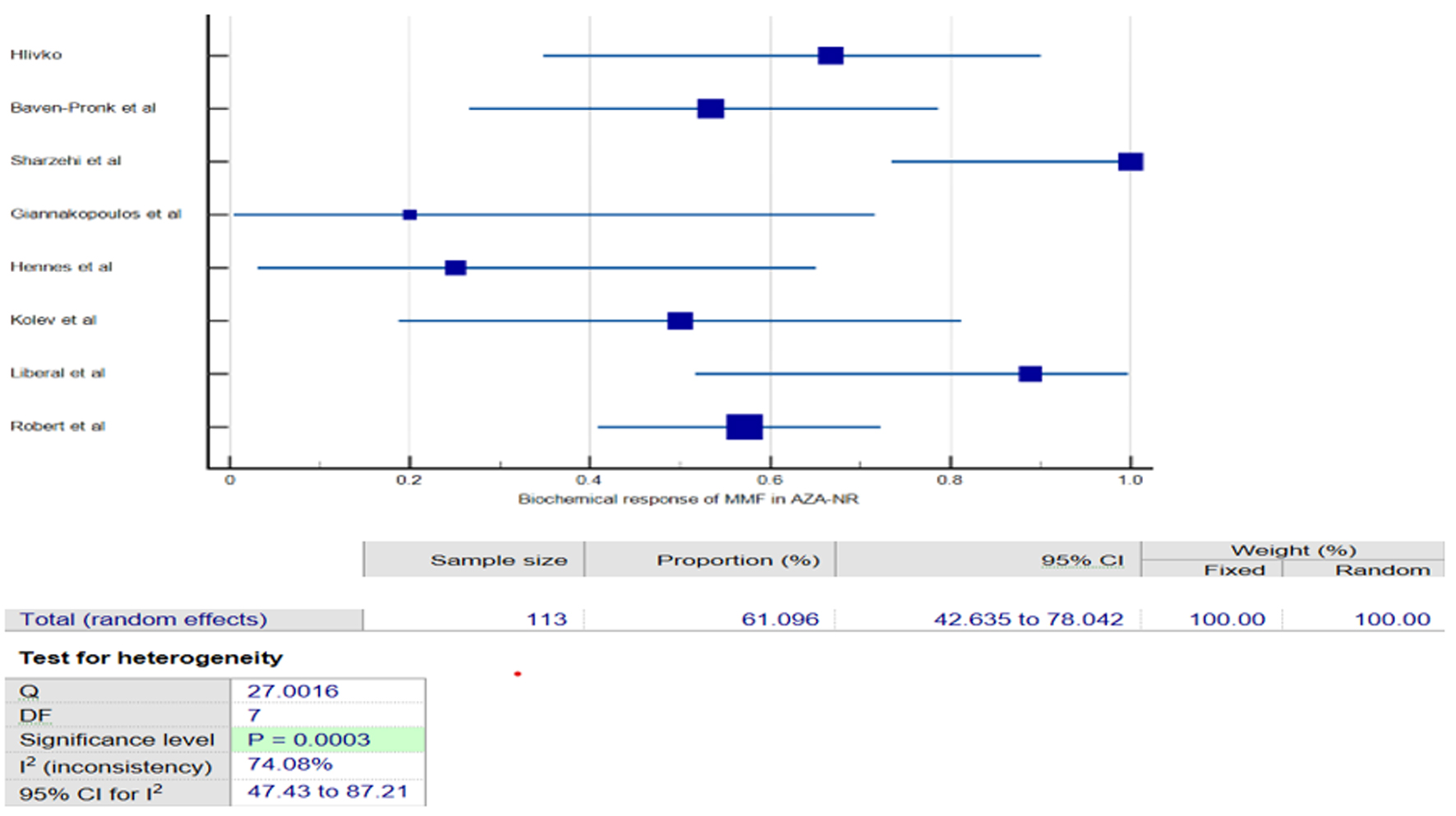 Click for large image | Figure 3. Forest plot of pooled biochemical remission of mycophenolate mofetil (MMF) in azathioprine non-respondents (AZA-NR). |
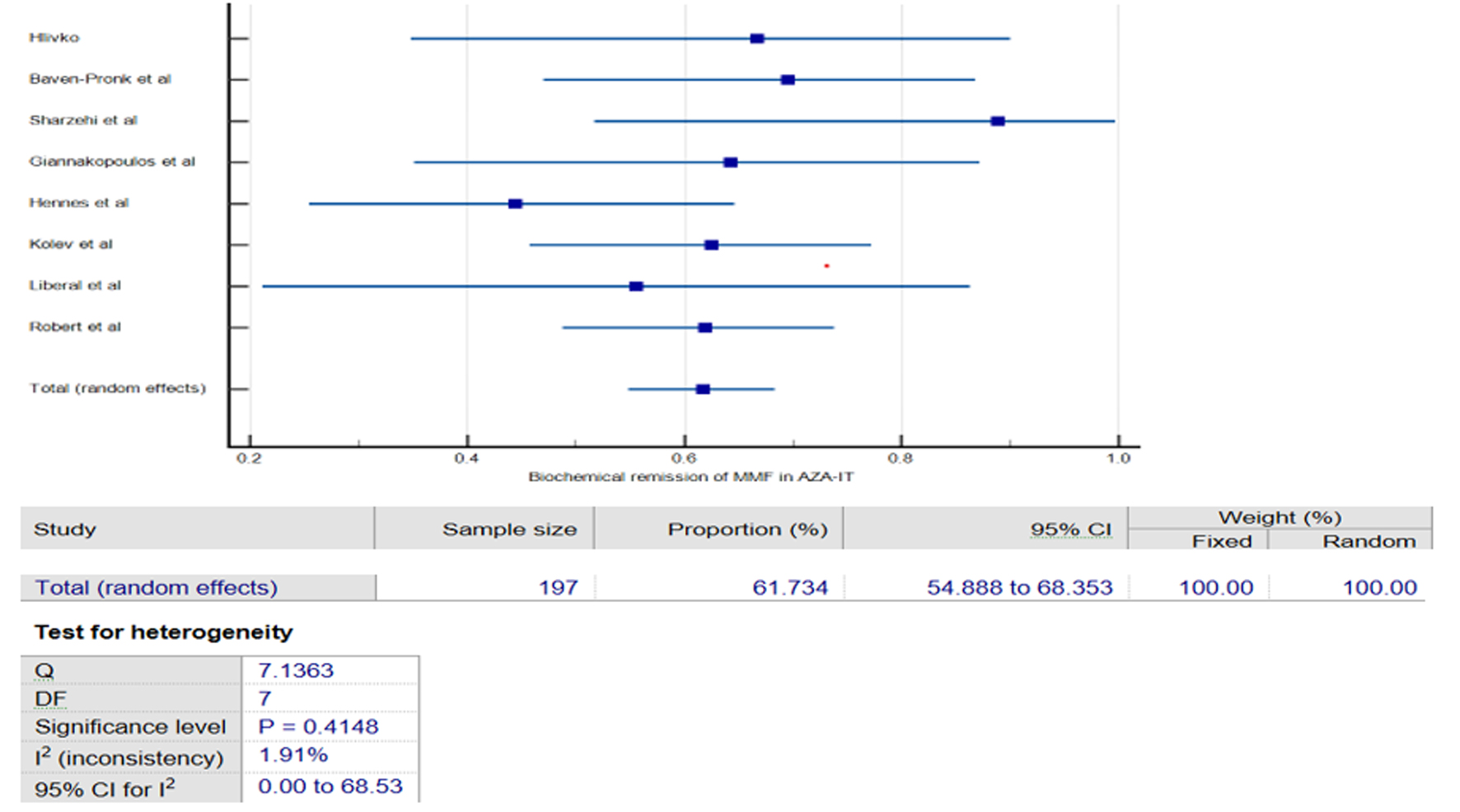 Click for large image | Figure 4. Forest plot of pooled biochemical remission of mycophenolate mofetil (MMF) in azathioprine intolerants (AZA-IT). |
AEs
This analysis did not reveal a statistically significant difference in the incidence of AEs between the MMF and AZA groups. The pooled OR for AEs was 0.57 (95% CI: 0.13 - 2.61, I2 = 86%, P = 0.47, Fig. 5), suggesting comparable safety profiles. However, in a sensitivity analysis that excluded the study by Hlivko et al due to its moderate risk of bias, the analysis demonstrated a lower rate of AEs with MMF compared to AZA (OR: 0.31, 95% CI: 0.10 - 0.98, I2 = 63%, P = 0.05). The AE data are summarized in Table 2. The most frequently reported AEs in these studies were gastrointestinal issues, infections, and fatigue. Importantly, the AZA group exhibited a higher incidence of hepatotoxicity than the MMF group, which did not report any case of hepatotoxicity. Furthermore, two patients in the MMF cohort developed malignancies, including lymphoma and melanoma, whereas no such events were reported in the AZA group [24]. Meta-analysis revealed a higher incidence of serious AEs necessitating treatment discontinuation in the AZA cohort (11.5%) than the MMF cohort (2.5%). Additionally, the pooled data demonstrated a lower risk of serious AEs with MMF than AZA (OR: 0.20, 95% CI: 0.08 - 0.46, I2 = 0%, P < 0.05). The AZA group experienced treatment-discontinuing AEs including hospitalization for drug-induced liver injury, fever, thrombocytopenia, and infections. The MMF group had severe peripheral edema and marked neutropenia, which led to discontinuation [23].
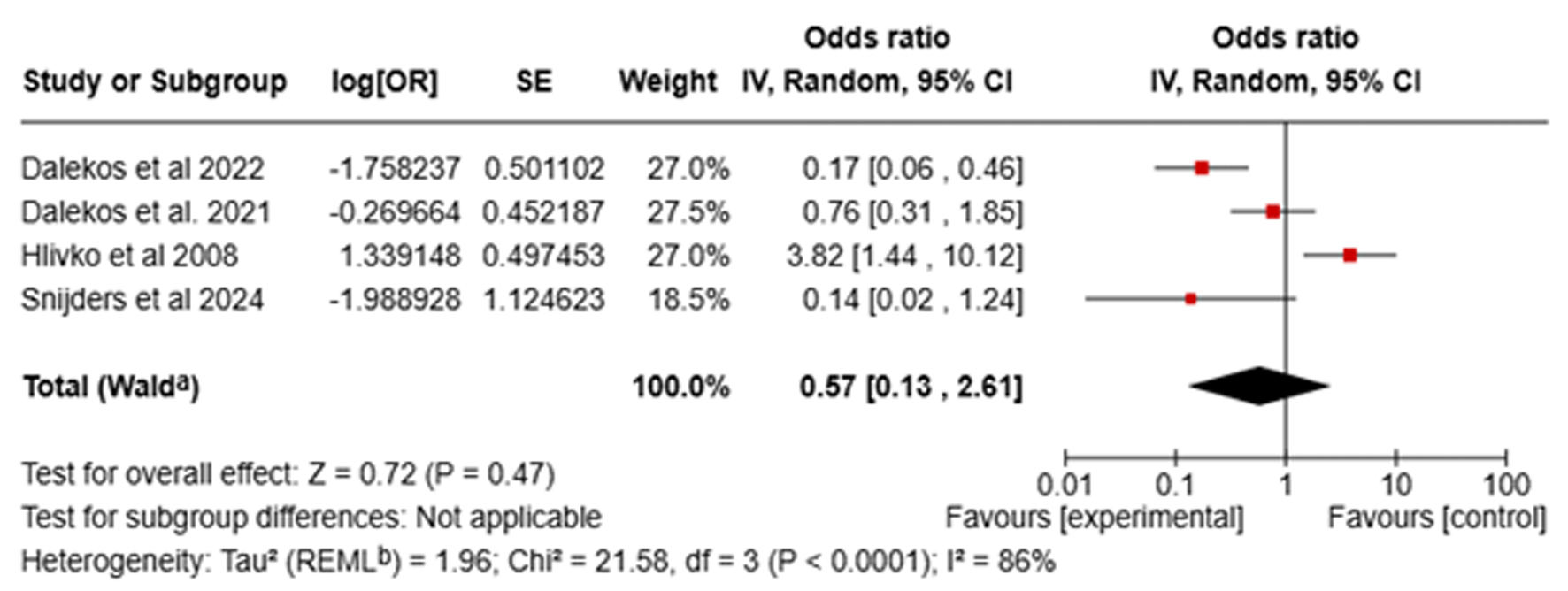 Click for large image | Figure 5. Forest plot of comparison of mycophenolate mofetil (MMF) with azathioprine (AZA) for adverse events. |
 Click to view | Table 2. Adverse Events |
Survival rate and steroid withdrawal
The analysis revealed no significant difference in overall survival between the MMF and AZA groups. The pooled OR for survival was 1.27 (95% CI: 0.47 - 3.41, I2 = 56%, P = 0.64), with moderate heterogeneity among the studies. Dalekos et al reported that relapse rates during corticosteroid tapering, or discontinuation were similar between MMF (37.8%) and AZA (36.1%) groups. By the end of the follow-up period, the majority of patients in both the MMF (74.6%) and AZA (76.6%) groups had successfully discontinued corticosteroids [22]. The need for the reintroduction of prednisolone was identical between the groups: 42% in the MMF group vs. 39% in the AZA group (P > 0.05) [22]. Additionally, the mean cumulative, daily, and weekly prednisolone doses were not significantly different between the two groups (P = 0.369) [23].
Quality of included studies
The methodological quality of the included studies was evaluated and the findings are presented in Supplementary Material 5 (gr.elmerpub.com). The Cochrane ROBINS-I tool was used to assess the risk of bias. Two independent reviewers (AA and MAK) assessed the risk of bias, with a third reviewer (SK) resolved any disagreements. The studies exhibited variability in quality, ranging from a low to moderate risk of bias. Specifically, three studies [21-23] were classified as having a low risk of bias, indicating a high degree of confidence in their findings, whereas seven studies [13-17, 19, 20] were identified as having a moderate risk of bias, suggesting some limitations in their design or execution. Only one study [18] was deemed to have a high risk of bias, primarily due to the small sample size and potential biases in the selection of participants and assessment of outcomes, which may compromise the validity of its conclusions.
| Discussion | ▴Top |
This systematic review and meta-analysis offers a rigorous comparative assessment of the therapeutic efficacy and safety profiles of MMF and AZA in the clinical management of AIH. These findings indicate that MMF demonstrates superior BR rates compared to AZA when utilized as a first-line therapy, particularly among AZA-IT or AZA-NR patients. Furthermore, the safety profiles of the two treatments were comparable, with no significant differences in the incidence of AEs. The substantial BR rate associated with MMF in this analysis is consistent with the conclusions drawn from previous meta-analyses, although those were constrained by factors such as the lack of direct head-to-head comparisons between the drugs and the limited availability of newer, higher-quality studies with extended follow-up periods [24, 25]. Additionally, more recent studies have incorporated the new response criteria and endpoints proposed by the International Autoimmune Hepatitis Group (IAIHG) [26].
The meta-analysis revealed that MMF achieved a significantly higher BR rate than AZA despite the high heterogeneity across studies. This study included both treatment-naive patients and those who received MMF as a subsequent intervention. Stratified analyses revealed consistent results across both treatment-naive patients and those treated with MMF as a second-line therapeutic strategy. The sensitivity analysis revealed that no single study disproportionately influenced the meta-analysis results. Heterogeneity in BR rates suggests variations in patients and clinical characteristics across studies. One study reported a sustained BR at 12 months favoring MMF, suggesting that it may provide longer-lasting improvements [22]. Sensitivity analysis limited to treatment-naive patients with AIH revealed that MMF demonstrated a sustained BR compared to AZA. This finding suggests that MMF may be a more effective initial treatment option for individuals newly diagnosed with AIH. Additionally, the meta-analysis incorporated a single RCT that reported higher remission rates with MMF than with AZA [23]. Snijders et al reported that after accounting for the presence of cirrhosis at the time of randomization, individuals treated with MMF were significantly more likely to attain BR at 24 weeks than those receiving AZA (OR: 3.57, P = 0.017). Dalekos et al reported comparable relapse rates and corticosteroid discontinuation between the MMF and AZA treatment arms. Additionally, the requirement for prednisolone reintroduction was similar, with no statistically significant differences observed in the mean prednisolone doses between the two groups [22].
Subgroup analyses revealed that MMF achieved a pooled BR rate of 61% among AZA-NR and a comparable remission rate of 61.73% in AZA-IT participants, with minimal heterogeneity observed in AZA-intolerant studies. Specifically, the AZA-NR subgroup experienced a 3.87% relapse rate and a 31.46% treatment failure rate with MMF, whereas the AZA-IT subgroup showed a 5.65% relapse rate and a 17.43% treatment failure rate with MMF. However, the data indicate that MMF may be less effective in patients who previously failed to respond to AZA, potentially reflecting broader refractoriness to immunosuppressive treatment in such cases. These results suggest that MMF may be an effective treatment option for individuals who are unable to tolerate or respond adequately to AZA-based therapy. Consequently, the Hellenic Association for the Study of the Liver has recommended MMF as a primary treatment option, particularly in specialized autoimmune hepatitis centers [27]. Roberts et al examined a cohort of cirrhotic AIH patients receiving MMF as second-line therapy. Their findings suggest lower response and higher failure rates in cirrhotic individuals, regardless of prior AZA intolerance or ineffectiveness. Despite the small sample size, the results imply that MMF may be less efficacious in decompensated liver disease despite similar tolerability [17]. This finding highlights the need for further research on MMF use in patients with cirrhotic AIH.
The analysis revealed comparable safety profiles between MMF and AZA, with no statistically significant difference in the incidence of AEs. Furthermore, excluding a study with a moderate risk of bias revealed fewer AEs with MMF [13]. The most common side effects include gastrointestinal issues, infections, and fatigue. Additionally, the analysis demonstrated a lower risk of serious AEs with MMF than AZA. The withdrawal rates due to adverse effects varied widely across studies, with Giannakopoulos et al reporting a 27% withdrawal rate for MMF, primarily due to gastrointestinal discomfort. Variability in AEs highlights the need for personalized patient management to mitigate side effects and optimize treatment adherence. MMF has a high teratogenic potential, so it should be avoided during pregnancy and only prescribed with strict contraception for women of childbearing age and men planning fatherhood, as it is absolutely contraindicated during pregnancy [28]. In contrast, AZA can be safely administered during pregnancy.
The meta-analysis revealed no statistically significant difference in overall survival between the MMF and AZA treatment groups. Similarly, Dalekos et al reported comparable overall survival and liver-related mortality rates between the two therapeutic approaches. However, their research identified several independent predictors associated with improved liver-related survival, including shorter disease duration, lack of cirrhosis or higher albumin levels at diagnosis, early diagnosis, and age at diagnosis under 60 years. Notably, the presence of cirrhosis at baseline emerged as a critical determinant of poor survival outcomes, corroborating existing evidence [14]. Additionally, a lower proportion of patients with cirrhosis achieved BR than those without cirrhosis (47% vs. 66%, P = 0.07) [17]. Decompensated liver cirrhosis, liver transplantation, and death were only observed in the AIH AZA-NR group (P < 0.001) [15]. These findings underscore the importance of early diagnosis and prompt initiation of effective therapy to enhance the survival of patients with AIH.
AIH overlap syndromes, which include conditions such as primary biliary cirrhosis (AIH-PBC) and primary sclerosing cholangitis (AIH-PSC), pose a distinct challenge owing to their rarity, resulting in limited knowledge regarding optimal treatment paradigms for this patient group [29]. Individuals with overlapping syndromes may present atypical biochemical profiles and exhibit variable responses to standard AIH treatments [30]. Typically, the AIH component in overlap syndromes is managed as AIH [31]. Therapeutic strategies for overlap syndromes are largely empirical, often combining steroids with ursodeoxycholic acid (UDCA) [32]. The IAIHG suggests that the management of overlap syndromes should be guided by predominant clinical manifestations [32]. Regimens targeting a single component of the overlap syndrome have shown efficacy in improving liver function tests in patients with either predominant AIH or cholestatic presentation [31]. Patients with AIH-PBC who do not fulfill the Paris criteria have demonstrated improvement with standard immunosuppressive therapy for AIH, whereas those with predominantly PBC and underlying features of AIH have benefited from UDCA alone [33]. Combination therapy has been correlated with improved laboratory results, stabilization of hepatic fibrosis, and preservation of 5-year transplant-free and 10-year overall survival in AIH-PBC patients [34]. However, treatment outcomes in adults with AIH-PSC have been inconsistent, with laboratory resolution occurring less frequently compared to AIH [31]. Furthermore, treatment failure and mortality due to liver failure or the necessity for liver transplantation have been more prevalent in AIH–PSC than in AIH [31]. The AASLD guidelines suggest considering the addition of UDCA to prednisone or prednisolone in combination with AZA in both adults and children with AIH and overlap syndromes [32]. In the included studies, the majority excluded individuals with AIH overlap syndrome, with only one study evaluating MMF in this specific patient population [15]. Among patients with overlap syndrome, remission was achieved in 57% and 63% of the AZA-NR and AZA-IT groups treated with MMF, respectively [15]. Notably, the study indicated that in AIH overlap syndrome patients, AZA non-response did not predict a non-response to MMF, unlike in AIH patients without overlap, although the patient population with overlap syndrome was relatively small [15]. In cases where first- and second-line treatments prove ineffective, anti-TNF and anti-CD20 therapies might be considered, although current data supporting their utilization remain limited [32, 35]. Rituximab, an anti-CD20 monoclonal antibody, has demonstrated potential for B-cell depletion and the management of refractory hepatic autoimmune overlap syndromes accompanied by autoimmune cytopenia [36, 37]. Belimumab, a B-lymphocyte stimulator inhibitor employed in systemic lupus erythematosus, may also represent a therapeutic option for patients with AIH exhibiting associated autoimmune characteristics [38]. Although calcineurin inhibitors have been employed in refractory cases, their associated side effect profiles may curtail their applicability [39]. Additional investigations are warranted to ascertain the optimal utilization of these alternative strategies within specific AIH patient subgroups.
Several limitations of the current evidence base merit acknowledgement. The included studies exhibited substantial heterogeneity, likely stemming from variations in the study design, patient populations, diverse geographic regions, outcome definitions, differences in dosing regimens, and disparate follow-up durations. Additionally, data on long-term outcomes and comparative efficacy of MMF versus AZA in maintaining remission are limited. Furthermore, the predominance of retrospective studies has introduced potential selection bias and confounding factors. This underscores the need for greater standardization of treatment protocols and reporting. Future research should explore the use of biomarkers to predict treatment responses and long-term outcomes. Personalized approaches considering patient-specific factors, such as baseline liver function, genetic predisposition, and comorbidities, could further optimize treatment strategies for AIH. The results of this analysis suggest that MMF may be a viable first-line therapeutic option for AIH, particularly in AZA-IT or AZA-NR patients. The findings indicate that MMF may be more effective in achieving both induction and maintenance of remission than standard AZA-based regimen, without compromising safety. From a cost-effectiveness standpoint, AZA generally remains less expensive than MMF, particularly in the US healthcare system, and is more widely covered by insurance plans [40]. However, a complete picture is more nuanced. Although the direct cost of MMF may be higher, especially for branded versions, this may be offset by other factors. For instance, some studies suggest that routine laboratory tests may be performed more frequently in AZA-treated patients, increasing costs [22]. In addition, MMF’s potentially superior tolerability could lead to fewer hospital admissions and days off due to side effects, further influencing overall cost-effectiveness [22]. Despite these potential offsets, the initial price difference can still create a significant barrier to access for patients without insurance or with high-deductible plans. Therefore, clinicians should carefully consider these economic factors along with efficacy and tolerability when making treatment decisions.
Conclusion
The meta-analysis results indicate that MMF may be a suitable primary treatment option for patients with AIH, especially those who are treatment-naive, intolerant, or non-responsive to AZA-based therapies. The analysis did not reveal any statistically significant differences in overall survival between the MMF and AZA treatment groups. Additionally, the safety profiles were comparable, with MMF demonstrating a lower risk of serious AEs, although the withdrawal rates varied considerably across the included studies. Nonetheless, further research is required to evaluate the long-term outcomes and develop personalized treatment approaches that account for patient-specific characteristics, which may optimize the management of AIH.
| Supplementary Material | ▴Top |
Suppl 1. Definitions of Biochemical Remission and Treatment Failure Across Studies.
Suppl 2. Full Search Strategy.
Suppl 3. PRISMA Flow Diagram of Included Studies.
Suppl 4. Outcomes Grading.
Suppl 5. Risk of Bias.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Institutional Review Board approval and informed consent were not required for this study.
Author Contributions
SK: concept, design, protocol development, literature search and review, statistical analysis, data interpretation, and draft. MAK: literature search and review, data extraction, review, and editing. AA: design, literature search and review, and editing. HA: literature search and review, data extraction, review, and editing. MS: literature search and review, data extraction, and review. AK: data extraction, review, and editing. MWE: data extraction, review, and editing. MPS: design, data interpretation, and critical revision of article. YZ: design, conceptualization (supporting), and critical revision of article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary information files.
Abbreviations
AH: autoimmune hepatitis; AZA: azathioprine; AZA-IT: azathioprine intolerant; AZA-NR: azathioprine non-respondent; IAHG: International Autoimmune Hepatitis Group; MMF: mycophenolate mofetil
| References | ▴Top |
- Reau NS, Lammert CS, Weinberg EM. Autoimmune hepatitis: Current and future therapies. Hepatol Commun. 2024;8(6):e0458.
doi pubmed - Kriese S, Heneghan MA. Current concepts in the diagnosis and management of autoimmune hepatitis. Frontline Gastroenterol. 2013;4(1):2-11.
doi pubmed - Theocharidou E, Heneghan MA. Current and future perspectives in autoimmune hepatitis. Br J Hosp Med (Lond). 2018;79(3):151-159.
doi pubmed - Mistilis SP, Skyring AP, Blackburn CR. Natural history of active chronic hepatitis. I. Clinical features, course, diagnostic criteria, morbidity, mortality and survival. Australas Ann Med. 1968;17(3):214-223.
doi pubmed - Schalm SW, Korman MG, Summerskill WH, Czaja AJ, Baggenstoss AH. Severe chronic active liver disease. Prognostic significance of initial morphologic patterns. Am J Dig Dis. 1977;22(11):973-980.
doi pubmed - Sucher E, Sucher R, Gradistanac T, Brandacher G, Schneeberger S, Berg T. Autoimmune hepatitis-immunologically triggered liver pathogenesis-diagnostic and therapeutic strategies. J Immunol Res. 2019;2019:9437043.
doi pubmed - Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A, Dalekos GN, et al. Review article: autoimmune hepatitis — current management and challenges. Aliment Pharmacol Ther. 2013;38(8):887-913.
doi pubmed - Schmeltzer PA, Russo MW. Clinical narrative: autoimmune hepatitis. Am J Gastroenterol. 2018;113(7):951-958.
doi pubmed - Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naive patients. J Hepatol. 2011;55(3):636-646.
doi pubmed - Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23(33):6030-6048.
doi pubmed - Pape S, Schramm C, Gevers TJ. Clinical management of autoimmune hepatitis. United European Gastroenterol J. 2019;7(9):1156-1163.
doi pubmed - Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
doi pubmed - Hlivko JT, Shiffman ML, Stravitz RT, Luketic VA, Sanyal AJ, Fuchs M, Sterling RK. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6(9):1036-1040.
doi pubmed - Giannakopoulos G, Verbaan H, Friis-Liby IL, Sangfelt P, Nyhlin N, Almer S, Swedish Hepatology study group S. Mycophenolate mofetil treatment in patients with autoimmune hepatitis failing standard therapy with prednisolone and azathioprine. Dig Liver Dis. 2019;51(2):253-257.
doi pubmed - Baven-Pronk AM, Coenraad MJ, van Buuren HR, de Man RA, van Erpecum KJ, Lamers MM, Drenth JP, et al. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther. 2011;34(3):335-343.
doi pubmed - Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol. 2010;24(10):588-592.
doi pubmed - Roberts SK, Lim R, Strasser S, Nicoll A, Gazzola A, Mitchell J, Siow W, et al. Efficacy and safety of mycophenolate mofetil in patients with autoimmune hepatitis and suboptimal outcomes after standard therapy. Clin Gastroenterol Hepatol. 2018;16(2):268-277.
doi pubmed - Liberal R, Gaspar R, Lopes S, Macedo G. Long-term outcome of patients with difficult-to-treat autoimmune hepatitis receiving mycophenolate mofetil. Clin Res Hepatol Gastroenterol. 2021;45(2):101487.
doi pubmed - Kolev M, Diem S, Diem L, Rodrigues SG, Berzigotti A, Stirnimann G, Semmo N. Mycophenolate mofetil as second line treatment in autoimmune hepatitis - A retrospective single center analysis. J Transl Autoimmun. 2022;5:100172.
doi pubmed - Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C, Kanzler S, et al. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103(12):3063-3070.
doi pubmed - Dalekos GN, Arvaniti P, Gatselis NK, Samakidou A, Gabeta S, Rigopoulou E, Koukoulis GK, et al. First results from a propensity matching trial of mycophenolate mofetil vs. azathioprine in treatment-naive AIH patients. Front Immunol. 2021;12:798602.
doi pubmed - Dalekos GN, Arvaniti P, Gatselis NK, Gabeta S, Samakidou A, Giannoulis G, Rigopoulou E, et al. Long-term results of mycophenolate mofetil vs. azathioprine use in individuals with autoimmune hepatitis. JHEP Rep. 2022;4(12):100601.
doi pubmed - Snijders R, Stoelinga AEC, Gevers TJG, Pape S, Biewenga M, Tushuizen ME, Verdonk RC, et al. An open-label randomised-controlled trial of azathioprine vs. mycophenolate mofetil for the induction of remission in treatment-naive autoimmune hepatitis. J Hepatol. 2024;80(4):576-585.
doi pubmed - Santiago P, Schwartz I, Tamariz L, Levy C. Systematic review with meta-analysis: mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49(7):830-839.
doi pubmed - Yu ZJ, Zhang LL, Huang TT, Zhu JS, He ZB. Comparison of mycophenolate mofetil with standard treatment for autoimmune hepatitis: a meta-analysis. Eur J Gastroenterol Hepatol. 2019;31(7):873-877.
doi pubmed - Pape S, Snijders R, Gevers TJG, Chazouilleres O, Dalekos GN, Hirschfield GM, Lenzi M, et al. Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J Hepatol. 2022;76(4):841-849.
doi pubmed - Dalekos GN, Papatheodoridis GV, Koskinas J, Goulis I, Rigopoulou EI, Tiniakos D, Hellenic Study Group for Autoimmune Liver Diseases of H. Hellenic Association for the Study of the Liver (HASL): revised clinical practice guidelines for autoimmune hepatitis. Ann Gastroenterol. 2024;37(6):623-654.
doi pubmed - Zullo F, Cardinale V, Alvaro D. Implications of mycophenolate mofetil in a reproductive age patient cohort: Maternal fetal medicine point of view. J Hepatol. 2025;82(1):e35-e36.
doi pubmed - Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14(21):3368-3373.
doi pubmed - Schultheiss C, Steinmann S, Willscher E, Paschold L, Lohse AW, Binder M. Immune signatures in variant syndromes of primary biliary cholangitis and autoimmune hepatitis. Hepatol Commun. 2023;7(5):e0123.
doi pubmed - Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E, International Autoimmune Hepatitis G. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54(2):374-385.
doi pubmed - Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the study of liver diseases. Hepatology. 2020;72(2):671-722.
doi pubmed - Joshi S, Cauch-Dudek K, Wanless IR, Lindor KD, Jorgensen R, Batts K, Heathcote EJ. Primary biliary cirrhosis with additional features of autoimmune hepatitis: response to therapy with ursodeoxycholic acid. Hepatology. 2002;35(2):409-413.
doi pubmed - Kuiper EM, Zondervan PE, van Buuren HR. Paris criteria are effective in diagnosis of primary biliary cirrhosis and autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol. 2010;8(6):530-534.
doi pubmed - Eldew H, Soldera J. Evaluation of biological therapies in autoimmune hepatitis: a case-based systematic review. World J Gastrointest Pathophysiol. 2025;16(1):101481.
doi pubmed - Appanna GD, Pembroke TPI, Miners KL, Price DA, Gallimore AM, Ladell K, Godkin AJ. Rituximab depletion of intrahepatic B cells to control refractory hepatic autoimmune overlap syndrome. QJM. 2019;112(10):793-795.
doi pubmed - Carey EJ, Somaratne K, Rakela J. Successful rituximab therapy in refractory autoimmune hepatitis and Evans syndrome. Rev Med Chil. 2011;139(11):1484-1487.
pubmed - Kolev M, Sarbu AC, Moller B, Maurer B, Kollert F, Semmo N. Belimumab treatment in autoimmune hepatitis and primary biliary cholangitis - a case series. J Transl Autoimmun. 2023;6:100189.
doi pubmed - Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, Moller S, et al. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58(3):529-534.
doi pubmed - James A, Mannon RB. The cost of transplant immunosuppressant therapy: is this sustainable? Curr Transplant Rep. 2015;2(2):113-121.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.