| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 5, October 2025, pages 207-223
Risk Stratification and Contemporary Predictors of Survival in Hepatocellular Carcinoma Treated With Transarterial Radioembolization
Deepak Sherpallya, g , Parthib Dasb, Fode Tounkarac, Khalid Mumtazd, Mina S. Makarye, Samuel Paulc, Eric Minc, Austin J. Simf, Anne Noonanb, Pannaga Malalurb, John Haysb, Ning Jinb, Arjun Mittrab, Eric Millerf, Dayssy Diaz Pardof, Kenneth Pitterf, Ashish Manneb
aDepartment of Internal Medicine, New York Medical College, Metropolitan, New York, NY 10029, USA
bDivision of Medical Oncology, Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, USA
cDepartment of Biomedical Informatics, Ohio State University, Columbus, OH 43210, USA
dDivision of Gastroenterology, Hepatology, and Nutrition, Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
eDepartment of Radiology, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
fDepartment of Radiation Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, USA
gCorresponding Author: Deepak Sherpally, Department of Internal Medicine, New York Medical College/Metropolitan, New York, NY 10029, USA
Manuscript submitted May 21, 2025, accepted July 28, 2025, published online October 9, 2025
Short title: Risk and Predictors in HCC Treated With TARE
doi: https://doi.org/10.14740/gr2049
| Abstract | ▴Top |
Background: Identifying risk factors for poor outcomes in patients with hepatocellular carcinoma (HCC) treated with transarterial radioembolization (TARE) can aid in developing personalized management strategies, such as the early use of immune checkpoint inhibitors (ICIs).
Methods: In this retrospective review, we included HCC patients who received TARE at The Ohio State University Comprehensive Cancer Center from January 1, 2015, to August 30, 2022. The Kaplan-Meier method was used to estimate progression-free survival (PFS) and overall survival (OS). Cox proportional hazard analysis was conducted to find the independent predictors of PFS and OS.
Results: We included 141 patients (median age of 65 years; 80% Caucasian; 80% male). Better PFS was associated with higher albumin (alb) (hazard ratio (HR) = 0.58, P = 0.005) and lower total bilirubin (T bili) levels (HR = 0.70, P = 0.034). Better OS was associated with a history of ablation (HR = 0.35, P < 0.001) and higher pre-TARE alb (HR = 0.63, P = 0.01); OS was worse in those with hepatic encephalopathy (HR = 2.01, P = 0.006). There was a notable trend toward worse OS in patients with ascites (HR = 1.71, P = 0.06) and metabolic-dysfunction-associated fatty liver disease (MAFLD)-associated HCC (HR = 1.86, P = 0.08). The receipt of ICI therapy was associated with a significantly better OS (P = 0.016), with a median OS of 1,102 days (95% confidence interval (CI): 884 - 1,509) compared to 614 days (95% CI: 493 - 829).
Conclusion: We present pretreatment risk factors (low alb, high T bili, MAFLD, hepatic encephalopathy, and ascites) that can predict poor outcomes in HCC patients treated with TARE. Preemptively treating such high-risk patients with ICI could improve their outcomes.
Keywords: Risk stratification; Hepatocellular carcinoma; Transarterial radioembolization; TARE; Liver function; Y90
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) is the most common liver cancer, accounting for more than 80% of primary liver tumors with a poor 5-year survival rate (about 18%), making it the third leading cause of cancer-related deaths worldwide [1-4]. Managing HCC necessitates collaboration between various disciplines, including hepatology, radiation oncology, nuclear medicine medical oncology, surgery, and interventional radiology. Many societies propose risk stratification guidelines for HCC treatment, primarily using those from the Barcelona Clinic Liver Cancer (BCLC) and the American Association for the Study of Liver Diseases (AASLD) [5, 6]. Clear guidelines for choosing the best locoregional therapies (LRTs) among transarterial chemoembolization (TACE), stereotactic body radiotherapy (SBRT), and transarterial radioembolization (TARE) for intermediate and non-metastatic advanced HCCs are lacking. It is often dependent on available expertise at the institution where the patient is treated.
TARE or selective internal radiation therapy (SIRT) refers to the deployment of 90Yttrium (Y90)-labeled microspheres directly into the tumor through the hepatic artery via a catheter [7, 8]. Microspheres used for TARE can be glass (TheraSphere, BTG International Group, London, UK) or resin (SIR-Spheres, Sirtex Medical Ltd, New South Wales, Australia) [9]. TARE was part of the AASLD guidelines (2018) and the National Comprehensive Cancer Network (NCCN) guidelines for quite some time but was featured in the BCLC guidelines only in the latest update (2022) [6, 10]. The economic burden limits its use in Asian countries even though it is part of the recommended guidelines [11]. In recent years, TARE has become a good option for LRT in the United States, supported by increasing evidence of its benefits and acceptable toxicity profiles for patients with early-to-intermediate-stage HCC with adequate liver function [12-14]. It is used with curative intent (segmentectomy) to deliver ablative doses to smaller tumors or for local disease control (lobectomy). However, its benefit over systemic therapy, such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), remains unclear in advanced tumors, as demonstrated in trials (SORAMIC, SARAH, and SIRveNIB) and limited retrospective studies [15-19].
In a recent study by Chen et al involving 413 patients with unresectable HCC, TARE microspheres achieved a median overall survival (OS) of 20.9 months (95% confidence interval (CI): 18.2 - 24.0). Notably, 17% of patients (n = 70) were able to undergo curative treatments following successful tumor downstaging. This subgroup exhibited a markedly improved median OS of 79.7 months (95% CI: 40.4 to not estimable), highlighting the potential of TARE as a bridge to curative therapy [20]. Additionally, recently published limited real-world data reported clinicopathological risk factors such as poor liver function (Child-Pugh B or C, low albumin, high total bilirubin (T bili), albumin-bilirubin (ALBI) grade 2 or higher), large tumor size, portal vein tumor thrombus (PVTT), high alfa-fetoprotein (AFP), and ascites as predictors of poor outcomes in patients with HCC treated with TARE [21-34]. The majority of these studies predated the era of ICI use in HCC. Furthermore, there are limited real-world data in the contemporary treatment era that integrate these risk factors with emerging systemic therapies involving ICIs.
In this retrospective study, we aimed to characterize pretreatment clinical predictors of outcomes (progression-free survival (PFS) and OS) in a contemporary cohort of patients with HCC treated with TARE at a large academic medical center. We also explored the impact of subsequent ICI therapy on survival outcomes and evaluated less commonly studied etiologies such as metabolic-dysfunction-associated fatty liver disease (MAFLD). Our findings offer new insights into risk stratification and raise the possibility that early ICI integration in select high-risk patients may improve outcomes.
| Materials and Methods | ▴Top |
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of The Ohio State University (OSU) (protocol number 2022C0177, approval date 12/11/2022). The requirement for informed consent was waived due to the retrospective nature of the study, which ensures no compromise to patient welfare or rights.
Patient selection and chart review
Patient electronic medical records (EMRs) from the OSU Wexner Medical Center (OSUWMC) from January 1, 2015, to August 30, 2022, were identified using the HCC diagnosis code from the International Statistical Classification of Diseases (ICD) (versions 9 and 10) and the current procedural terminology (CPT) procedure code for TARE. All eligible patients were discussed in our institutional multidisciplinary liver tumor board (LTB) meeting, and a unanimous decision was made to offer TARE. Patients included in this study were diagnosed with HCC based on imaging or biopsy and received at least one session of TARE during the study period. We excluded patients with HCC who underwent TARE and then received liver transplants to avoid competing risks. Planned staged procedures (without any disease progression after the first TARE session) were taken as one procedure for analysis.
Data extracted from the EMR included demographics (age, race, and gender), tumor-related clinicopathological data (etiology of cirrhosis such as MAFLD, viral hepatitis, autoimmune liver disease, etc.), smoking history, alcohol use history, ascites, hepatic encephalopathy (HE), and prior and post-progression therapy received including LRTs and systemic therapy. We also recorded HCC characteristics, including location, size and number of lesions, PVTT, and relevant laboratory data (T bili, albumin, AFP at the time of diagnosis, and international normalized ratio (INR)). Other treatments received (LRTs and systemic therapy) before and after TARE were also retrieved.
Outcomes
The primary outcomes were PFS, OS, and OS specific to TARE (OS-TARE). PFS was defined as the time between the TARE procedure (the first, if the patient had multiple procedures) and disease progression noted on the scans, death, or the last date of follow-up. OS was defined as the interval from the date of diagnosis to the date of death or the last follow-up date. OS-TARE was defined as the interval from the date of the first TARE procedure to the date of death or the last date of follow-up. This distinction between OS and OS-TARE was made to isolate the survival benefit attributable to TARE and subsequent therapies (OS-TARE), while also accounting for the influence of prior treatments on overall survival from diagnosis (OS).
Post-TARE contrast-enhanced imaging results, in the form of either computed tomography (CT) or magnetic resonance imaging (MRI) reports available after the procedure, were reviewed in the LTB meeting to assess the response to the procedure using modified Response Evaluation Criteria in Solid Tumors (mRECIST). Most patients underwent CT scans, and few had MRIs. We also reported the impact of TARE on albumin, T bili, and Child-Pugh score (during follow-up to assess treatment response).
Statistical analysis
A statistical analysis evaluated the potential association between the exposures of interest (gender, race, baseline laboratory values, and clinical data) and different survival outcomes such as PFS, OS, and OS-TARE. Patients who did not die were censored at the date of the last follow-up.
The Kaplan-Meier method was employed to estimate PFS, OS, and OS-TARE. Univariate and Cox proportional hazard analyses assessed the association between exposures and various survival outcomes (PFS, OS, and OS-TARE). The log-rank test was utilized for categorical variables, and the Cox proportional hazard was used for continuous variables. A paired t-test was used to compare the pre- and post-TARE changes in serum albumin, T bili, and Child-Pugh scores.
| Results | ▴Top |
Baseline characteristics
Our initial EMR search resulted in 175 patients; we excluded 20 patients with colorectal metastasis in the liver and 14 patients with HCC who proceeded to have liver transplants, leaving 141 patients eligible for this study. Baseline characteristics are shown in Table 1. Most of our patients were Caucasian (80%), and males (80%) dominated the study population. The group’s median age was 65 (range 49 - 88) years. Ascites (25%) and HE (29%) were the two most common decompensations of cirrhosis. The majority (n = 122; 87%) of the patients had one session of TARE followed by two sessions (n = 17; 12%) and three sessions (n = 2; 1%). TARE was the initial therapy in 50 patients (35.46%). However, the rest of the patients received other LRTs, including TACE (n = 59; 41.84%), ablation (35; 24.82%), surgery (n = 18; 12.77%), SBRT (n = 7; 4.97%), and systemic therapy (12; 8.52%) prior to TARE. Most of the study patients had one HCC lesion (n = 65; 46.10%) followed by two lesions (n = 30; 21.28%). Interestingly, HCC tumor sizes were ≤ 3 cm and > 3 cm in 56 (40%) and 79 (56%) patients, respectively, with PVTT in 24 patients and the majority having Child-Pugh score A (n = 105; 74.47%).
 Click to view | Table 1. Baseline Characteristics of the Study Population (N = 141) |
Among those who underwent TARE, only 39 patients were on ICIs. Five patients received TARE after HCC progression while on ICIs (pre-TARE ICI group), and 34 received ICIs after HCC progression on TARE (post-TARE ICI group). One patient in the pre-TARE group continued ICI treatment after TARE. Around 65% of the study population had TARE as the second or third line of management. The most common ICI used was a combination of atezolizumab and bevacizumab in 20 (51%) patients, followed by nivolumab alone in 19 (49%).
Factors impacting PFS
The median PFS of our patients was 129 days (CI: 105 - 171 days). Table 2 outlines the unadjusted (univariate) and adjusted (multivariate) hazard ratios (HRs) for various factors influencing PFS in the study population.
 Click to view | Table 2. Factors Impacting PFS in the Study Population Treated With TARE |
Lower serum bilirubin (HR = 0.70, CI: 0.50 - 0.97, P = 0.034) and higher serum albumin (HR: 0.58, CI: 0.40 - 0.85, P = 0.005) were the independent predictors of PFS among patients treated with TARE. Patients with MAFLD (HR = 2.13, CI: 1.10 - 4.13, P = 0.06) and prior TACE (HR = 1.38, P = 0.08) had an increased risk of progression, though these trends did not reach statistical significance in multivariate analysis. Similarly, ascites and HE suggested an increased progression risk but lacked statistical significance in the adjusted model. Notably, HCC with left PVTT was associated with a significant reduction in progression risk in univariate analysis (HR = 0.22, P = 0.038). It is important to note that we studied the impact of the specific location of PVTT (left, right, and main portal vein) for univariate analysis only for PFS, OS, and OS-TARE. The presence of PVTT (yes vs. no) was used for multivariate analysis.
Impact of ICI on PFS
Most patients in our study did not receive ICI before TARE. Their PFS was worse than that of the small pre-TARE ICI group (median PFS of 128 days (95% CI: 106 - 171 days) vs. (393 days (95% CI: 100 to not evaluable (NE)), suggesting that ICI use before TARE might delay disease progression significantly. However, the wide CI and the lower bound starting at 100 days suggest substantial variability among patients. The impact of ICI before TARE had no significant impact on PFS in univariate analysis (HR = 0.64, 95% CI: 0.26 - 1.58, P = 0.3) or multivariate analysis (HR = 0.90, 95% CI: 0.32 - 2.51, P = 0.8).
Factors impacting OS-TARE
The group’s median OS-TARE is 311 days (CI: 248 - 438 days). We outlined the factors influencing TARE (unadjusted (univariate) and adjusted (multivariate) HRs) (Table 3).
 Click to view | Table 3. Factors Impacting TARE-Specific OS in the Study Population |
Higher albumin levels pre-TARE were significantly correlated with improved OS-TARE in both analyses. Conversely, a history of MAFLD or HE were associated with worse OS-TARE outcomes in both analyses. Additional findings revealed that a history of left PVTT was associated with improved OS-TARE in univariate analysis. While ascites at the time of TARE appeared to impact OS-TARE in univariate analysis negatively, this association was not statistically significant in the multivariate model when controlling for other factors.
The influence of ICI on OS-TARE
Patients who received ICI treatment (pre- or post-TARE groups) had a better (P = 0.0005) OS-TARE than those who did not receive ICIs (697 days (95% CI: 609 - 1,022 days) vs. 226 days (95% CI: 200 - 308 days)) (Fig. 1a). The post-TARE ICI group had a significantly (P = 0.0026) better OS-TARE than the pre-TARE ICI group (703 days (95% CI: 609 - 1,022 days) vs. 678 days (95% CI: 393 - NE)) (Fig. 1b). ICI post-TARE was associated with improved OS-TARE in both univariate and multivariate analyses.
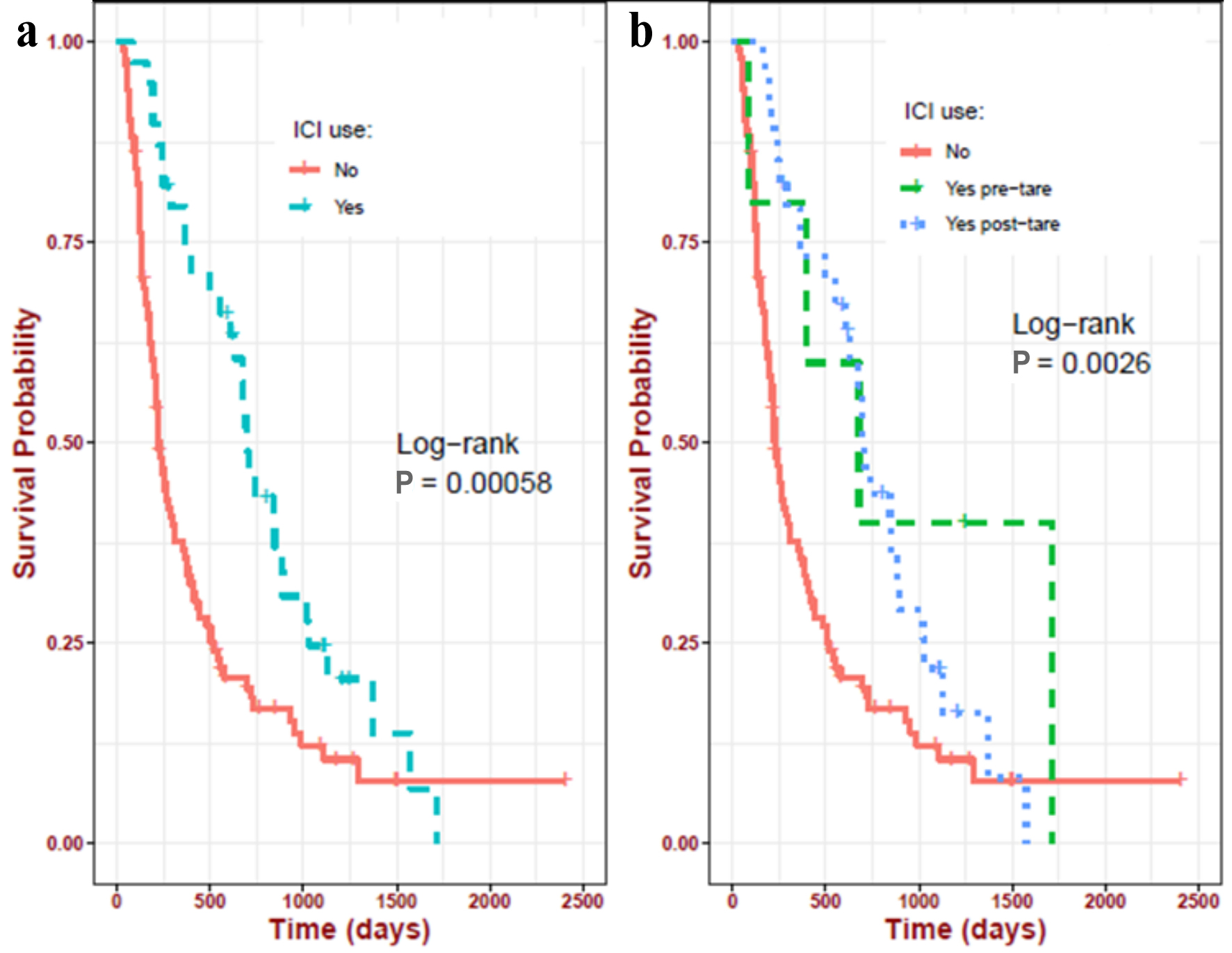 Click for large image | Figure 1. Impact of ICIs on the TARE-related OS in the study population. (a) ICI at any time in their lifetime (697 days) vs. never received ICI (226 days); (b) ICI before TARE (678 days) vs. ICI after TARE (703 days) vs. never received ICI (226 days). ICIs: immune checkpoint inhibitors; OS: overall survival; pre-TARE: ICI before TARE; post-TARE: ICI after TARE; TARE: transarterial radioembolization. |
Factors impacting OS
The study population’s OS was 765 days (CI: 614 - 941 days). Table 4 provides a comprehensive overview of both univariate and multivariate HRs for various factors affecting OS.
 Click to view | Table 4. Factors Impacting OS in the Study Population |
Demographic factors such as age and race did not significantly influence OS in adjusted models. MAFLD substantially increased mortality risk in the univariate model (HR = 2.23, P = 0.004) and remained a strong risk factor in the adjusted model (HR = 1.86, P = 0.082). HE significantly increased mortality risk in both univariate (HR = 1.61, P = 0.016) and multivariate (HR = 2.01, P = 0.006) analyses, indicating a critical impact on survival. Ascites increased mortality risk, with multivariate analysis showing HR = 1.71 (P = 0.065), approaching statistical significance. TARE used in patients with a history of ablation had a better outcome (univariate HR = 0.50, P < 0.001; multivariate HR = 0.35, P < 0.001), highlighting its benefit in this population. Higher albumin levels were significantly associated with better survival outcomes (multivariate HR = 0.63, P = 0.019), emphasizing the importance of nutritional and liver function status in patient prognosis.
The influence of ICI on OS
Median OS significantly (P = 0.016) improved (1,102 days (95% CI: 884 - 1,509 days) vs. 614 (95% CI: 493 - 829 days)) in patients treated with ICIs, suggesting a substantial OS benefit from ICI use (Fig. 2a). In the former group, the pre-TARE ICI group (1,959 days (95% CI: 1,557 - NE)) experienced better survival than the post-TARE ICI group (927 days (95% CI: 755 - 1,270 days)) as illustrated in Figure 2b, and the difference was significant (pre-TARE ICI > post-TARE ICI > no ICI groups, P = 0.038). This differs from OS-TARE (post-TARE ICI > pre-TARE ICI > vs. no ICI groups). ICI before or after TARE did not significantly impact univariate or multivariate analysis. However, on univariate analysis, there was a trend toward improving survival with ICI before (HR = 0.39, P = 0.067) and after (HR = 0.65, P = 0.059) TARE.
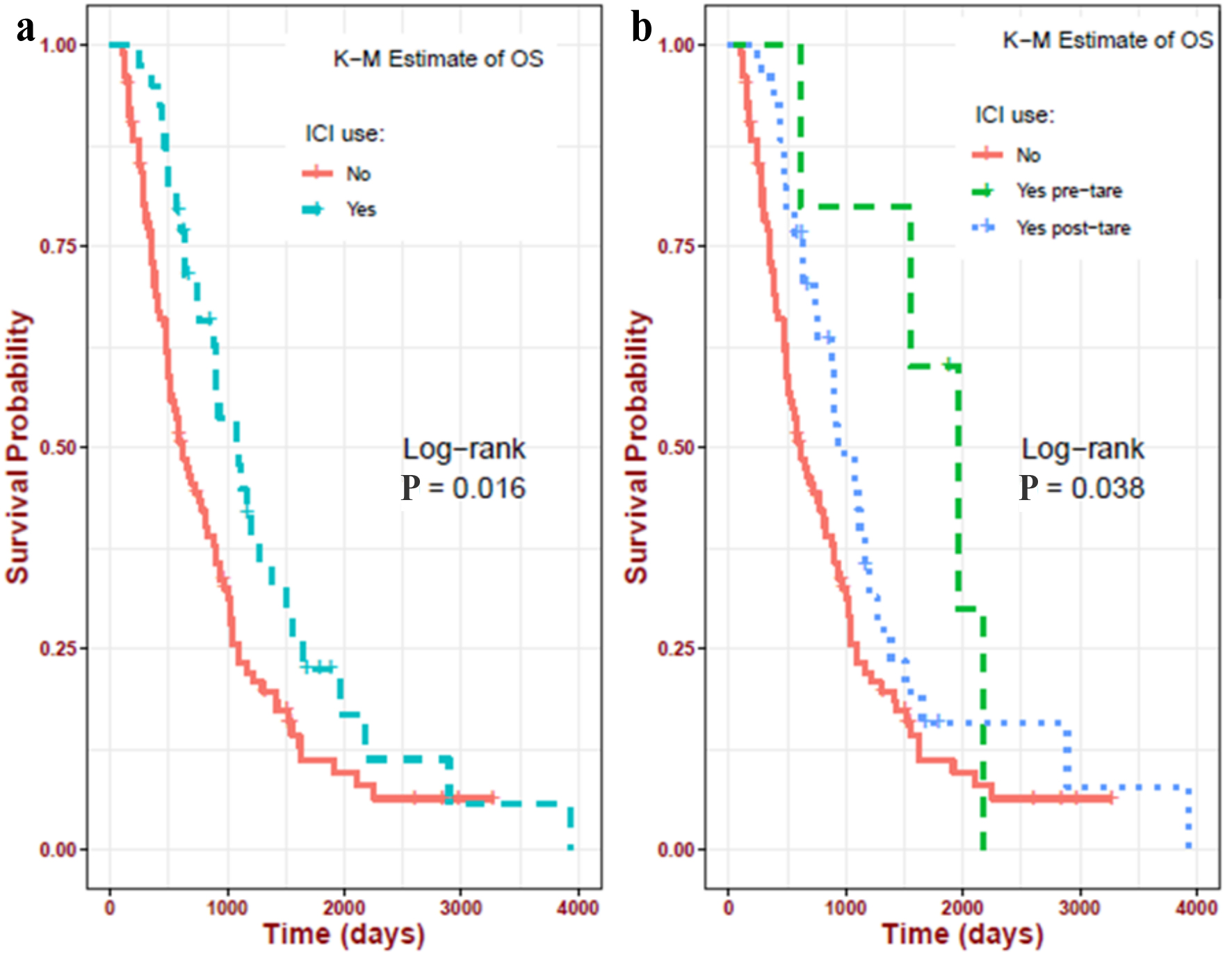 Click for large image | Figure 2. Impact of ICIs on the OS in the study population. (a) ICI at any time in their lifetime (1,102 days) vs. never received ICI (614 days); (b) ICI before TARE (1,959 days) vs. ICI after TARE (927 days) vs. never received ICI (614 days). ICIs: immune checkpoint inhibitors; OS: overall survival; pre-TARE: ICI before TARE; post-TARE: ICI after TARE; TARE: transarterial radioembolization. |
Comparing the patients who received ICI therapy
We compared the ICI group (n = 39, pre-TARE + post-TARE ICI groups) and the no-ICI group (Supplementary Material 1, gr.elmerpub.com). The ICI group tended to have a high fraction of patients pre-treated with surgery (23% vs. 9%, P = 0.04) or SBRT (18% vs. 6%, P = 0.04) and TKIs before or after TARE (47% vs. 17%, P < 0.001) as compared to the no ICI group. The overall functional status of the liver of ICI group patients was better in the ICI post-TARE group, as demonstrated by significantly (P = 0.04) higher BCLC A cases (56% vs. 35%) and fewer BCLC B (39% vs. 48%) and BCLC C (5% vs. 17%). This is supported by a strong trend (P = 0.05) toward improvement in the mean T bili level (in mg/dL, 1 (standard deviation (SD) 1.7) vs. 2 (2.5), P = 0.02) in the ICI group. The albumin levels were negligibly better in the ICI group, too (in mg/dL, 3 (0.6) vs. 3 (0.5), P = 0.002). Similar results were seen when we compared the pre-TARE ICI, post-TARE ICI, and no ICI groups (Supplementary Material 2, gr.elmerpub.com). Those in the pre-TARE ICI group tended to receive LRTs before TARE such as surgery (pre-TARE vs. post-TARE vs. no ICI = 80% vs. 15% vs. 9%, respectively, P < 0.01) or SBRT (40% vs. 15% vs. 6%, respectively, P = 0.02), and tended to receive TKIs before TARE (40% vs. 6% vs. 3%, respectively, P = 0.014) or any time during their life (80% vs. 38% vs. 17%, respectively, P < 0.01). The mean albumin levels in the post-TARE ICI group were higher than in other groups.
Impact of TARE on liver function
We compared key indicators of liver function, albumin, total bilirubin, and Child-Pugh scores before and after TARE to study liver toxicity of the procedure (Table 5).
 Click to view | Table 5. Impact of TARE on Liver Function |
Albumin levels increased significantly after the procedure, with a mean increase of 0.53 g/dL. The CI does not contain zero, reinforcing that the change is statistically significant. This suggests that the procedure has a positive effect on the albumin levels of patients, possibly indicating improved liver synthetic function. T bili levels decreased significantly after the procedure, with a mean decrease of 0.91 mg/dL. The CI, again not containing zero, confirms the statistical significance of this decrease. This indicates that the procedure effectively reduces T bili levels, suggesting improved liver function related to bilirubin processing. The Child-Pugh scores, which assess the prognosis of chronic liver disease, primarily cirrhosis, showed a significant decrease, with a mean reduction of 1.36 points. This substantial decrease indicates an overall improvement in liver function or reduced severity of liver disease following the procedure.
Imaging and outcome
We did not have follow-up imaging reports for 11 patients (one died, and the reports were unavailable to verify response in 10 others). Among the 130 patients with imaging, the median follow-up imaging took place at 83 days after TARE (second TARE in patients who had two pre-planned procedures). Thirty-nine (39) patients (30%) had clear-cut progression of disease (PD), while 48% (n = 63) had an objective response, with complete response (CR) in 18% (n = 23) and partial response (PR) in 31% (n = 40). The reports were ambiguous (AR, no clear evidence of response or progression) in 22% (n = 28). The PFS was best among those with CR (median PFS of 303 days, 95% CI: 213 - 985) compared to those with PR (143 days, 95% CI: 106 - 254) or PD (80 days, 95% CI: 106 - 254). Patients with AR (132 days, 95% CI: 93 - 201) had PFS between PR and PD (AR < PR < PD). The difference among these groups (Fig. 3) was significant (P < 0.001).
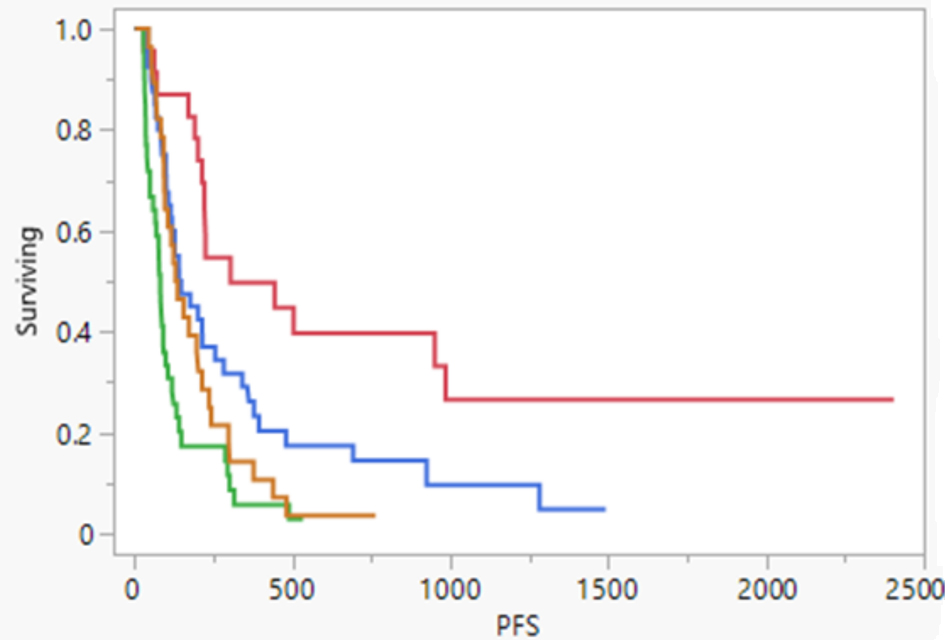 Click for large image | Figure 3. Comparing PFS in patients based on the response noted in the first post-TARE imaging scan. Red indicates complete response, green indicates disease progression, blue indicates partial response, and yellow indicates ambiguous response. PFS: progression-free survival; TARE: transarterial radioembolization. |
| Discussion | ▴Top |
There is an urgent need to improve the dismal outcomes associated with HCC. The traditional approach of waiting for treatment failure with procedures such as TARE could lead to a worsening of the patient’s physical condition (ECOG performance status (PS)) or liver function (Child-Pugh B or C), deeming them ineligible for most LRTs or systemic therapy. The current approval for atezolizumab and bevacizumab is only for patients with good liver function (Child-Pugh A) based on the IMbrave 150 study [35]. Even though durvalumab and tremelimumab is approved irrespective of Child-Pugh score, its clinical benefit in Child-Pugh B and C patients is unknown [36]. Identifying patients at risk of TARE failure will enable treating physicians to implement effective strategies to improve outcomes.
Our study provides valuable insights into TARE’s real-world utilization and outcomes in managing HCC, particularly in the context of patients receiving both TARE and ICI, a treatment that is attracting much interest. The factors we incorporated in our multivariate analysis are more comprehensive than most studies published. We intentionally avoided classifications such as BCLC in our analysis, as its definition is not uniform in all guidelines (AASLD vs. BCLC) and it is not strictly followed at most American institutes when selecting LRTs.
The impact of prior ICI on PFS was negligible. On the other hand, patients who received ICI at any time (pre- or post-TARE ICI groups) had significantly better outcomes (OS and OS-TARE) than the no-ICI group. Unlike typical cancer treatment analyses, interpreting OS-TARE and OS and their influencing factors is complex. Analyzing OS-TARE provides insight into the factors affecting outcomes following TARE. Traditional OS offers an understanding of influences before and after TARE. This dual perspective is essential since 65% of patients had undergone prior therapies, including various LRTs, ICI, and TKI. The median OS was significantly better in the pre-TARE ICI group by a large margin of 1,023 days (about 34 months or 3 years). In contrast, the median OS-TARE was negligibly (25 days) better in the post-TARE ICI group.
This underscores two major confounding factors that need to be considered: the intrinsic aggressiveness of HCC, which may be linked to genetic factors such as mutations or methylation along with clinicopathological factors, and the impact of LRTs and systemic therapies, specifically ICI, on TARE effectiveness. Although we lacked tissue or blood-based genomic correlatives to determine the impact of LRTs on survival, we explored the influence of clinicopathological features by including an extensive list of factors in our multivariate analysis. This included specific LRTs (surgery vs. TACE vs. SBRT vs. ablation) and systemic therapies (TKI, ICI). The ICI group (pre- or post-TARE) in our study had a higher fraction of patients who were pretreated with definitive procedures such as surgery or SBRT; received TKI (pre- or post-TARE); had improved T bili and albumin levels post-TARE; and whose disease was classified as BCLC A after TARE (Supplementary Materials 1 and 2, gr.elmerpub.com). These findings suggest that careful patient selection is crucial for enhancing TARE outcomes, and incorporating ICI may play a significant role in this process.
Notably, ICI was not administered as part of standard-of-care (SOC) treatment before or after TARE; rather, it was given after disease progression post-TARE, or TARE was applied to patients who had progressed on ICIs. Nonetheless, this suggests that combining ICI with TARE may synergistically delay disease progression, potentially by enhancing the immune-mediated effects of TARE, as supported by translational evidence [37]. Chew et al showed that tumor-infiltrating lymphocytes (TILs) isolated post-TARE showed signs of immune activation (higher expression of granzyme B (GB) and infiltration of CD8+ T cells, CD56+ NK cells, and CD8+ CD56+ NKT cells), and next-generation sequencing showed up-regulation of relevant genes [37]. This is similar to the abscopal effect with combined SBRT and ICI, which we discussed at length in our previous publication [38]. There are encouraging data from early-phase clinical trials suggesting the safety and efficacy of this combination [39-43]. Multiple trials are currently underway to further investigate the benefit of adding ICI to TARE (EMERALD-Y90 or NCT06040099, NCT03099564, NCT04541173, NCT05063565) that could give us answers on the timing and sequencing of ICI to optimize clinical outcomes [44].
Our study also highlighted several other prognostic factors to consider when selecting patients for TARE (Table 6).
 Click to view | Table 6. Factors Influencing Survival in HCC Patients Treated With TARE in Our Study |
Some risk factors influencing OS, such as HE, ascites, lower T bili, and higher albumin levels at the time of TARE, are not surprising as they reflect overall liver health and are consistent with previous reports available in the literature [21, 23, 24, 31]. Patients with prior ablation are typically under active surveillance, which may lead to earlier detection of recurrence with favorable features, such as smaller tumor size and preserved liver function, making them suitable candidates for TARE. This underscores the critical role of maintaining hepatic reserve in HCC management, as patients with better liver function are more likely to tolerate and benefit from TARE and other therapeutic interventions. As reflected in Table 1 and Supplementary Materials 1 and 2 (gr.elmerpub.com), most patients did not have concerning T bili or albumin levels. Therefore, we interpret this as patients with T bili levels at the higher end of normal or albumin levels at the lower end of normal are at the risk of having poor TARE-related outcomes.
The MAFLD population in our study is low (12%), but those who had it experienced lower OS and PFS; however, in another study, it did not negatively impact outcomes [45]. MAFLD has previously been associated with aggressive HCC biology, potentially due to an immune activation, impaired autophagy, and genetic mutations, including CTNNB1 and TP53, contribute to the progression of MAFLD to HCC, with dysregulated pathways such as Wnt/β-catenin and Ras/MAPK playing key roles [46, 47]. As the prevalence of MAFLD-related HCC continues to rise, identifying effective treatment strategies for this patient population will be important. The factors impacting OS-TARE (albumin levels, MAFLD, and HE) or PFS (total bilirubin and albumin) were not that different from the factors impacting OS. We did not see any convincing evidence of the negative impact of PVTT on outcomes as reported in other studies [26, 27, 31]. On univariate analysis, history of a main PVTT had a negative impact on OS (HR = 1.84, P = 0.041) while patients with left PVTT had a good OS-TARE (HR = 0.21, P = 0.031) or PFS (HR = 0.22, P = 0.038). The poor outcomes observed in patients with main PVTT in our study are consistent with existing literature and may be attributed to more severely compromised portal blood flow in this subgroup [22, 48]. However, the small number of patients with left PVTT (n = 4) limits our ability to draw definitive conclusions regarding outcome differences between left, right, and main PVTT. Larger studies incorporating detailed assessments of liver function, including markers of inflammation, fibrosis, and portal hypertension, are needed to validate these findings and better delineate the clinical implications of PVTT location on TARE outcomes.
Following TARE, the observed improvements in liver function parameters, such as increased albumin and decreased bilirubin levels, further highlight its clinical benefits. This may be attributed to the localized cytotoxic effect of TARE, which induces effective tumor cell death while sparing normal hepatic tissue, thereby preserving and, in some cases, enhancing liver function. This is important with regard to tolerability and maintaining treatment eligibility, especially with potential combinations with systemic agents. Finally, patients who do not show clear evidence of response with TARE in the first post-procedural scans should be considered as having a PR and be treated accordingly. This approach may include initiating systemic therapy or pursuing other lines of treatment. We need to consider incorporating new tools to better assess early treatment response to TARE, such as evaluating enhancing tumor volume (ETV) in post-TARE MRI (≥ 65% reduction in ETV is associated with improved survival) or tumor vascularity in contrast to enhanced ultrasound 2 weeks post-TARE (reduction associated with response) [49, 50].
Limitations of this study include its retrospective nature and the potential for selection bias in the patient population. Additionally, the various treatment approaches and the relatively small sample size for specific subgroup analyses may limit the generalizability of the findings. For most of the study period (2015 - 2022), ICI was not the SOC. Atezolizumab and bevacizumab were approved for use in the clinic in mid-2021, and durvalumab/tremelimumab was much later. Even though single-agent nivolumab and pembrolizumab were available in 2020, their use was not favored in most populations as sorafenib and lenvatinib were still SOC [51-53]. Hence, we need more recent real-world data to understand the benefit of ICI with TARE. We acknowledge several technical differences in TARE, including the doses of radiation delivered, the choice between lobectomy and segmentectomy, and the number of TAREs performed on each patient (staged and retreatment). Patient selection was typically through a multidisciplinary discussion. Physician bias could have played a role in selecting TARE for patients. Pre-TARE or post-TARE AFP levels were not available to us for most patients, so we could not study its impact on treatment response or survival, though it has been reported in some other studies [25, 30]. Similarly, though we had patient ECOG scores at diagnosis and post-TARE follow-up visits, we did not have them just before TARE, which would have enhanced our study. Finally, the study did not incorporate genomic or molecular biomarkers that dictate tumor biology and could provide insights into HCC aggressiveness or modulate therapeutic response to TARE and other treatment modalities. Nonetheless, these real-world data provide valuable insights that can inform clinical decision-making and help optimize patient selection for TARE in HCC management.
Future research should focus on several critical areas. First, prospective trials are needed to define the optimal timing and sequencing of ICIs with TARE, particularly considering our findings suggesting differential survival benefits based on treatment order. Second, translational studies integrating tissue and blood-based biomarkers (for example, immune gene expression profiles, circulating tumor DNA, and immune cell phenotyping) are essential to identify predictive markers of response to TARE-ICI combinations. Third, there is a need to evaluate functional imaging biomarkers, such as early changes in ETV on MRI or vascularity shifts on contrast-enhanced ultrasound, to refine early response assessment and identify patients at risk of treatment failure. Fourth, the differential impact of HCC etiology, especially MAFLD-associated HCC, which emerged as a potential negative prognostic factor in our cohort, warrants further investigation given its rising global incidence and possible implications for immune response and disease biology. Lastly, standardized protocols for TARE delivery, including dosing strategies, segmental vs. lobar administration, and retreatment criteria, should be systematically evaluated across real-world settings to better define their influence on outcomes and harmonize practice. As immunotherapy becomes increasingly central to HCC treatment paradigms, clarifying how LRTs like TARE can be integrated in a personalized, biomarker-driven framework will be essential for improving outcomes in this complex and heterogeneous disease.
Conclusion
In this large real-world cohort, TARE demonstrated favorable safety and efficacy profiles in patients with HCC, particularly when integrated into a multimodal treatment approach that included ICIs. Our findings highlight the importance of preserved liver function and prior liver-directed therapies in shaping treatment outcomes and suggest that post-TARE immunotherapy (including ICI) may confer significant survival benefits. These results underscore the need for prospective studies to optimize sequencing strategies, incorporate molecular and functional biomarkers for better patient selection, and define the role of TARE in the evolving immunotherapy landscape of HCC.
| Supplementary Material | ▴Top |
Suppl 1. Comparing groups that received immune checkpoint inhibitor therapy and transarterial radioembolization (TARE) in the study population.
Suppl 2. Comparing groups that received immune checkpoint inhibitor therapy before and after transarterial radioembolization (TARE) in the study population.
Acknowledgments
The authors would like to thank Angela Dahlberg, editor in The Ohio State University Comprehensive Cancer Center Division of Medical Oncology, for editing this manuscript. Biorender.com was used for preparing figures.
Financial Disclosure
No relevant financial disclosures.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Author Contributions
Conceptualization, methodology, data curation, supervision, and writing - draft supervision: Ashish Manne. Writing - original draft preparation: Deepak Sherpally. Data curation and writing- review and editing: Parthib Das. Data curation: Samuel Paul and Eric Min. Biostatistics/formal analysis: Fode Tounkara. Writing - review and editing: Fode Tounkara, Khalid Mumtaz, Mina Makary, Austin J. Sim, Anne Noonan, Pannaga Malalur, John Hays, Ning Jin, Arjun Mittra, Eric Miller, Dayssy Diaz Pardo, and Kenneth Pitter.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary information files.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477-491.e471.
doi pubmed - Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345-1362.
doi pubmed - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922-1965.
doi pubmed - Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, Kelley RK, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681-693.
doi pubmed - Badar W, Yu Q, Patel M, Ahmed O. Transarterial radioembolization for management of hepatocellular carcinoma. Oncologist. 2024;29(2):117-122.
doi pubmed - Hamad A, Aziz H, Kamel IR, Diaz DA, Pawlik TM. Yttrium-90 Radioembolization: Current Indications and Outcomes. J Gastrointest Surg. 2023;27(3):604-614.
doi pubmed - Saini A, Wallace A, Alzubaidi S, Knuttinen MG, Naidu S, Sheth R, Albadawi H, et al. History and evolution of yttrium-90 radioembolization for hepatocellular carcinoma. J Clin Med. 2019;8(1):55.
doi pubmed - Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
doi pubmed - Cho Y, Kim BH, Park JW. Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: An Asian perspective comparison. Clin Mol Hepatol. 2023;29(2):252-262.
doi pubmed - Kim E, Sher A, Abboud G, Schwartz M, Facciuto M, Tabrizian P, Knesaurek K, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol. 2022;7(9):843-850.
doi pubmed - Dhondt E, Lambert B, Hermie L, Huyck L, Vanlangenhove P, Geerts A, Verhelst X, et al. (90)Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE phase II randomized controlled trial. Radiology. 2022;303(3):699-710.
doi pubmed - Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology. 2021;74(5):2342-2352.
doi pubmed - Ricke J, Klumpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, Gasbarrini A, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71(6):1164-1174.
doi pubmed - Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913-1921.
doi pubmed - Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624-1636.
doi pubmed - Jeschke M, Ludwig JM, Leyh C, Pabst KM, Weber M, Theysohn JM, Lange CM, et al. Bilobar radioembolization carries the risk of radioembolization-induced liver disease in the treatment of advanced hepatocellular carcinoma: safety and efficacy comparison to systemic therapy with atezolizumab/bevacizumab. Cancers (Basel). 2023;15(17):4274.
doi pubmed - Agirrezabal I, Bouattour M, Pinato DJ, D'Alessio A, Brennan VK, Carion PL, Shergill S, et al. Efficacy of transarterial radioembolization using Y-90 resin microspheres versus atezolizumab-bevacizumab in unresectable hepatocellular carcinoma: A matching-adjusted indirect comparison. Eur J Cancer. 2024;196:113427.
doi pubmed - Chen K, Tong AKT, Moe FNN, Ng DCE, Lo RHG, Gogna A, Yan SX, et al. The impact of radiation dose and tumour burden on outcomes in hepatocellular carcinoma: 11-year experience in a 413-patient cohort treated with yttrium-90 resin microsphere radioembolisation. Liver Cancer. 2025;14(2):158-179.
doi pubmed - Goswami P, Adeniran OR, S KF, Matsuoka L, Du L, Gandhi RT, Collins ZS, et al. Overall survival and toxicity of Y90 radioembolization for hepatocellular carcinoma patients in Barcelona Clinic Liver Cancer stage C (BCLC-C). BMC Gastroenterol. 2022;22(1):467.
doi pubmed - Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52-64.
doi pubmed - Antkowiak M, Gabr A, Das A, Ali R, Kulik L, Ganger D, Moore C, et al. Prognostic role of albumin, bilirubin, and ALBI scores: analysis of 1000 patients with hepatocellular carcinoma undergoing radioembolization. Cancers (Basel). 2019;11(6):879.
doi pubmed - Bayona Molano MDP, Kolber M, Barrera JV, Akram MR, Alnablsi MW, Pothini T, Salem R, et al. Prognostic value of liver biomarkers in hepatocellular carcinoma patients undergoing yttrium 90 transarterial radioembolization (TARE): a retrospective pilot study. Cureus. 2024;16(6):e61904.
doi pubmed - Bhutiani N, O'Brien SJ, Priddy EE, Egger ME, Hong YK, Mercer MK, McMasters KM, et al. Correlating serum alpha-fetoprotein in hepatocellular carcinoma with response to Yttrium-90 transarterial radioembolization with glass microspheres (TheraSphere). HPB (Oxford). 2020;22(9):1330-1338.
doi pubmed - Lee HM, Alder L, Nguyen M, Dougherty SC, Qu Y, Thacker LR, Poklepovic A. Long-term outcome analysis of Y90 radioembolization in hepatocellular carcinoma. J Gastrointest Oncol. 2023;14(3):1378-1391.
doi pubmed - Gordon AC, Gabr A, Riaz A, Uddin OM, Abouchaleh N, Ali R, Kallini J, et al. Radioembolization super survivors: extended survival in non-operative hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2018;41(10):1557-1565.
doi pubmed - Floridi C, Pesapane F, Angileri SA, De Palma D, Fontana F, Caspani F, Barile A, et al. Yttrium-90 radioembolization treatment for unresectable hepatocellular carcinoma: a single-centre prognostic factors analysis. Med Oncol. 2017;34(10):174.
doi pubmed - Shah RM, Sheikh S, Shah J, Vivian E, Mejia A, Shahin I, Mantry PS. Prognostic factors of unresectable hepatocellular carcinoma treated with yttrium-90 radioembolization: results from a large cohort over 13 years at a single center. J Gastrointest Oncol. 2021;12(4):1718-1731.
doi pubmed - Ekmekcioglu O, Tabakci ON, Bozdag Kaplan N, Hasanefendioglu Bayrak A, Battal M. Factors affecting the response to treatment and survival in hepatocellular carcinoma patients treated with transarterial radioembolisation: a single-centre experience. Eur J Gastroenterol Hepatol. 2021;33(6):926-931.
doi pubmed - Kolligs F, Arnold D, Golfieri R, Pech M, Peynircioglu B, Pfammatter T, Ronot M, et al. Factors impacting survival after transarterial radioembolization in patients with hepatocellular carcinoma: Results from the prospective CIRT study. JHEP Rep. 2023;5(2):100633.
doi pubmed - Finessi M, Cioffi M, Grimaldi S, Fronda M, Rovera G, Passera R, Carucci P, et al. Albi score predicts overall survival (OS) in patients with hepatocellular carcinoma (HCC) treated with selective internal radiation therapy (SIRT). Radiol Med. 2025;130(2):271-279.
doi pubmed - Chaikajornwat J, Tanasoontrarat W, Phathong C, Pinjaroen N, Chaiteerakij R. Clinical outcome of Yttrium-90 selective internal radiation therapy (Y-90 SIRT) in unresectable hepatocellular carcinoma: experience from a tertiary care center. Liver Res. 2022;6(1):30-38.
doi pubmed - Frantz S, Matsuoka L, Vaheesan K, Petroziello M, Golzarian J, Wang E, Gandhi R, et al. Multicenter evaluation of survival and toxicities of hepatocellular carcinoma following radioembolization: analysis of the RESiN registry. J Vasc Interv Radiol. 2021;32(6):845-852.
doi pubmed - Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862-873.
doi pubmed - Sangro B, Chan SL, Kelley RK, Lau G, Kudo M, Sukeepaisarnjaroen W, Yarchoan M, et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2024;35(5):448-457.
doi pubmed - Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, Lai L, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68(2):335-346.
doi pubmed - Jiang J, Diaz DA, Nuguru SP, Mittra A, Manne A. Stereotactic body radiation therapy (SBRT) plus immune checkpoint inhibitors (ICI) in hepatocellular carcinoma and cholangiocarcinoma. Cancers (Basel). 2022;15(1):50.
doi pubmed - Garcia-Reyes K, Gottlieb RA, Menon KM, Bishay V, Patel R, Patel R, Nowakowski S, et al. Radioembolization plus immune checkpoint inhibitor therapy compared with radioembolization plus tyrosine kinase inhibitor therapy for the treatment of hepatocellular carcinoma. J Vasc Interv Radiol. 2024;35(5):722-730.e721.
doi pubmed - de la Torre-Alaez M, Matilla A, Varela M, Inarrairaegui M, Reig M, Lledo JL, Arenas JI, et al. Nivolumab after selective internal radiation therapy for the treatment of hepatocellular carcinoma: a phase 2, single-arm study. J Immunother Cancer. 2022;10(11).
doi pubmed - Fabritius MP, Ricke J. Overview of ongoing clinical trials on radioembolization. Cardiovasc Intervent Radiol. 2022;45(11):1659-1662.
doi pubmed - Lee YB, Nam JY, Cho EJ, Lee JH, Yu SJ, Kim HC, Paeng JC, et al. A phase I/IIa trial of yttrium-90 radioembolization in combination with durvalumab for locally advanced unresectable hepatocellular carcinoma. Clin Cancer Res. 2023;29(18):3650-3658.
doi pubmed - Kaya NA, Tai D, Lim X, Lim JQ, Lau MC, Goh D, Phua CZJ, et al. Multimodal molecular landscape of response to Y90-resin microsphere radioembolization followed by nivolumab for advanced hepatocellular carcinoma. J Immunother Cancer. 2023;11(8):e007106.
doi pubmed - Su YY, Liu YS, Hsiao CF, Hsu C, Chen LT. Trial designs for integrating novel therapeutics into the management of intermediate-stage hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:517-536.
doi pubmed - Brunson C, Struycken L, Schaub D, Ref J, Goldberg D, Hannallah J, Woodhead G, et al. Comparative outcomes of trans-arterial radioembolization in patients with non-alcoholic steatohepatitis/non-alcoholic fatty liver disease-induced HCC: a retrospective analysis. Abdom Radiol (NY). 2024;49(8):2714-2725.
doi pubmed - Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
doi pubmed - Daher D, Dahan KSE, Singal AG. Non-alcoholic fatty liver disease-related hepatocellular carcinoma. J Liver Cancer. 2023;23(1):127-142.
doi pubmed - Qadan M, Kothary N, Sangro B, Palta M. The treatment of hepatocellular carcinoma with portal vein tumor thrombosis. Am Soc Clin Oncol Educ Book. 2020;40:1-8.
doi pubmed - Ludemann W, Kahn J, Pustelnik D, Hardt J, Boning G, Jonczyk M, Amthauer H, et al. Yttrium-90 radioembolization for unresectable hepatocellular carcinoma: predictive modeling strategies to anticipate tumor response and improve patient selection. Eur Radiol. 2022;32(7):4687-4698.
doi pubmed - Delaney LJ, Tantawi M, Wessner CE, Machado P, Forsberg F, Lyshchik A, O'Kane P, et al. Predicting long-term hepatocellular carcinoma response to transarterial radioembolization using contrast-enhanced ultrasound: initial experiences. Ultrasound Med Biol. 2021;47(9):2523-2531.
doi pubmed - Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
doi pubmed - Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173.
doi pubmed - Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77-90.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.