| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 5, October 2025, pages 232-238
Efficacy of Potassium-Competitive Acid Blockers in Functional Dyspepsia: A Systematic Review and Meta-Analysis
Hao The Nguyena, c , Arman Vaghefib, Tassawwar Khana
aCollege of Osteopathic Medicine, Rocky Vista University, Parker, CO, USA
bDepartment of Internal Medicine, University of New Mexico, Albuquerque, NM, USA
cCorresponding Author: Hao The Nguyen, College of Osteopathic Medicine, Rocky Vista University, Parker, CO, USA
Manuscript submitted June 30, 2025, accepted August 21, 2025, published online October 9, 2025
Short title: Efficacy of PCABls in FD
doi: https://doi.org/10.14740/gr2059
| Abstract | ▴Top |
Background: Functional dyspepsia (FD) is a prevalent gastrointestinal disorder characterized by upper abdominal discomfort, often refractory to standard acid suppression therapy. Potassium-competitive acid blockers (PCABs), such as vonoprazan and tegoprazan, provide rapid and sustained gastric acid inhibition and may represent a therapeutic alternative. However, their efficacy in FD remains unclear. This systematic review and meta-analysis aimed to evaluate the effect of PCAB therapy on symptom outcomes in adults with FD.
Methods: A systematic search of PubMed, EMBASE, and Cochrane CENTRAL was conducted from inception to June 13, 2025, to identify studies evaluating PCABs in adult FD patients. Eligible studies included randomized controlled trials and prospective or retrospective cohorts reporting validated symptom scores before and after PCAB therapy. Studies were included in meta-analysis if they reported 4-week outcomes with sufficient data for effect size calculation. Standardized mean differences (SMDs) were pooled using a DerSimonian-Laird random-effects model. Heterogeneity was assessed with the I2 statistic, and robustness was evaluated with leave-one-out sensitivity analysis and Baujat plots.
Results: Five studies comprising 366 patients were included. Four treatment arms from three studies (n = 276) were eligible for meta-analysis. The pooled SMD for symptom improvement at 4 weeks was 1.09 (95% confidence interval (CI): 0.67 - 1.52), indicating a moderate-to-large treatment effect in favor of PCABs. Heterogeneity was substantial (I2 = 77.8%), but sensitivity analyses showed that no single study unduly influenced results. The remaining two studies, excluded from quantitative pooling due to incompatible timepoints or outcome structures, also demonstrated statistically significant symptom improvement, supporting consistency of effect across the evidence base.
Conclusion: PCABs may offer clinically meaningful symptom relief in FD, with pooled data suggesting a moderate-to-large effect size. While heterogeneity and limited sample size temper generalizability, findings were consistent across studies and robust to sensitivity testing. PCABs show promise as a potential therapeutic option in FD, particularly in patients who do not respond to or cannot tolerate proton pump inhibitors. Further placebo-controlled trials are needed to confirm efficacy and define long-term outcomes.
Keywords: Functional dyspepsia; Potassium-competitive acid blockers; Vonoprazan; Tegoprazan; Acid suppression
| Introduction | ▴Top |
Functional dyspepsia (FD) is a condition characterized by a constellation of symptoms affecting the upper gastrointestinal system including epigastric pain or burning, early satiety, and epigastric fullness [1]. The prevalence of this condition has been estimated from 8.4% to 16% in otherwise healthy individuals with risk factors including psychiatric comorbidities, acute gastroenteritis, smoking, non-steroidal anti-inflammatory drug (NSAID) use, and Helicobacter pylori (H. pylori) infection [1, 2]. The pathophysiology is multifactorial, involving visceral hypersensitivity, impaired gastric accommodation, and disordered gastric motility [3]. Due to its multi-mechanistic causes, it is difficult to fully treat this condition though interventions such as dietary intervention, H. pylori eradication, and proton pump inhibitors (PPIs) have shown to be effective. Alternative therapies, such as cognitive behavioral therapy, have also been gaining recognition in recent years [4].
Potassium-competitive acid blockers (PCABs), including vonoprazan and tegoprazan, represent a newer class of acid suppressants that offer rapid, potent, and sustained intragastric pH control. Their role is well established in conditions such as erosive esophagitis and H. pylori eradication, but their effectiveness in FD remains uncertain.
Although several clinical studies have evaluated PCABs in FD, findings are limited by small sample sizes, variation in outcome measures, and inconsistent reporting. No prior synthesis has quantitatively assessed their impact on symptom outcomes in this population. This systematic review and meta-analysis aimed to evaluate the current evidence on PCAB therapy in FD.
| Materials and Methods | ▴Top |
Study design and registration
This study was conducted as a systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [5]. The research question, inclusion criteria, and analytic plan were determined prior to the execution of the search and adhered to a standardized protocol throughout the review process.
Search strategy
We systematically searched PubMed (MEDLINE), EMBASE, and the Cochrane Central Register of Controlled Trials from database inception through June 13, 2025. The search used a combination of controlled vocabulary and free-text terms related to FD and PCABs, including specific agents such as vonoprazan and tegoprazan. Search strategies were adapted for each database, and Boolean operators were used as appropriate. No language or publication date restrictions were applied. Reference lists of included studies and relevant reviews were manually screened to identify additional eligible articles. Duplicate records were removed prior to screening.
Eligibility criteria
We included studies that met the following criteria: 1) Population: adults (≥ 18 years) with FD, defined by Rome criteria or comparable clinical standards. 2) Intervention: PCAB monotherapy or combination therapy. 3) Outcomes: pre- and post-treatment symptom scores using validated dyspepsia instruments. 4) Design: randomized controlled trials, prospective cohorts, and retrospective studies.
Exclusion criteria included pediatric populations, studies without extractable symptom data, or those assessing PCABs solely for gastroesophageal reflux disease (GERD) without distinguishing dyspepsia outcomes.
Study selection and data extraction
Two reviewers independently screened titles, abstracts, and full texts for eligibility. Discrepancies were resolved by a third reviewer. Extracted data included study design, population size, country, diagnostic criteria, PCAB type/dose/duration, outcome measures, and symptom scores before and after treatment.
Statistical analysis
Meta-analysis was performed for studies reporting symptom outcomes at approximately 4 weeks using standardized mean differences (SMDs) under a DerSimonian-Laird random-effects model. When multiple doses were studied, each arm was analyzed independently. Heterogeneity was assessed using the I2 statistic, with values > 50% considered substantial. To evaluate the robustness of the pooled estimate, a leave-one-out sensitivity analysis was conducted, and results were visualized using an influence plot. A Baujat plot was also generated to assess the relative contribution of individual studies to overall heterogeneity and their influence on the pooled effect size. Studies unsuitable for pooling were summarized narratively. All analyses were conducted using Python (v3.11). Forest plots were used to display individual and pooled SMDs with 95% confidence intervals (CIs).
| Results | ▴Top |
Study selection
The search yielded 159 records. After duplicate removal and screening, 10 full text articles were reviewed. Five studies met inclusion criteria and were included in the analysis. The study selection process is detailed in Figure 1.
 Click for large image | Figure 1. PRISMA flow diagram outlining the study selection process. |
Study characteristics and outcomes
Five studies comprising 366 adults with FD evaluated the therapeutic effect of PCABs [6-10]. Four studies assessed vonoprazan (10 - 20 g) and one evaluated tegoprazan (50 mg). Symptom outcomes were measured using validated instruments including the Nepean Dyspepsia Index (NDI), Modified Global Overall Symptom Scale (GOSS), Gastrointestinal Symptom Rating Scale (GSRS), and Izumo Scale. Most studies assessed symptoms at 4 weeks; one study followed participants for 24 weeks and another provided data points at 8 weeks.
All five studies reported statistically significant improvement in dyspepsia symptoms following PCAB therapy (P < 0.001). Four treatment arms across three studies were eligible for meta-analysis. Two studies were excluded from pooling: one due to a longer follow-up window (24 weeks) and lack of pooled outcome scoring, the other due to non-comparable reporting of symptom scores, though both showed directional consistency. A summary of these five studies can be seen in Table 1 [6-10].
 Click to view | Table 1. Characteristics of Included Studies Evaluating Potassium-Competitive Acid Blockers in Functional Dyspepsia |
Pooled analysis
Four treatment arms from three studies (n = 276) reporting 4-week symptom outcomes were included in the meta-analysis. SMDs were used to account for variation in symptom assessment tools. The pooled SMD was 1.09 (95% CI: 0.67 - 1.52), indicating a moderate-to-large treatment effect favoring PCAB therapy (Fig. 2).
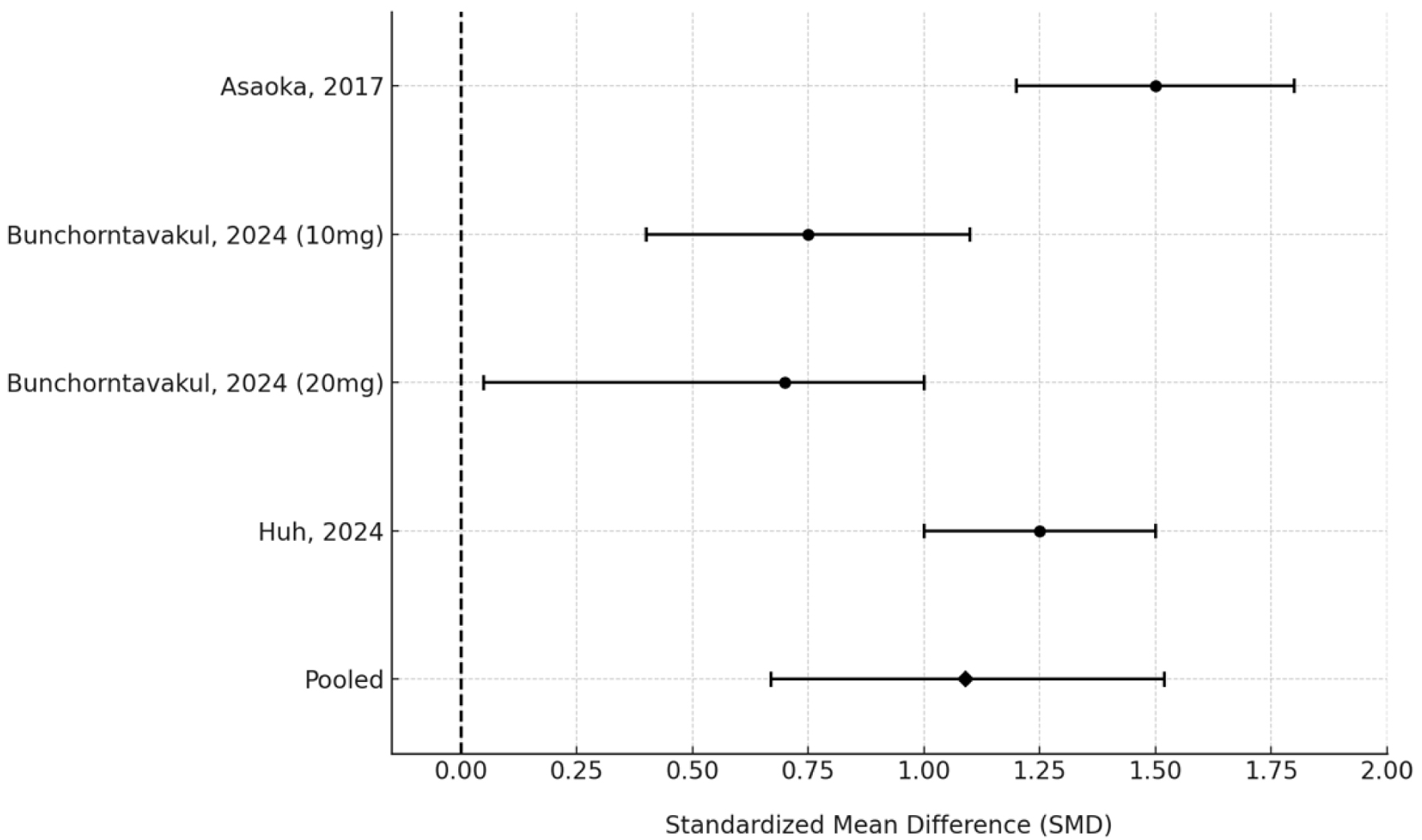 Click for large image | Figure 2. Forest plot of standardized mean differences in symptom scores following 4-week PCAB therapy across included studies. PCAB: potassium-competitive acid blocker. |
Heterogeneity remained high (I2 = 77.8%, τ2 = 0.14), likely due to differences in outcome scales, patient populations, and PCAB dosing regimens. Sensitivity analysis using a leave-one-out approach confirmed the robustness of the pooled estimate, with no single study driving the result (Fig. 3). A Baujat plot was used to visualize study-specific contributions to heterogeneity and effect size (Fig. 4); while some variation was observed, no study significantly distorted the findings.
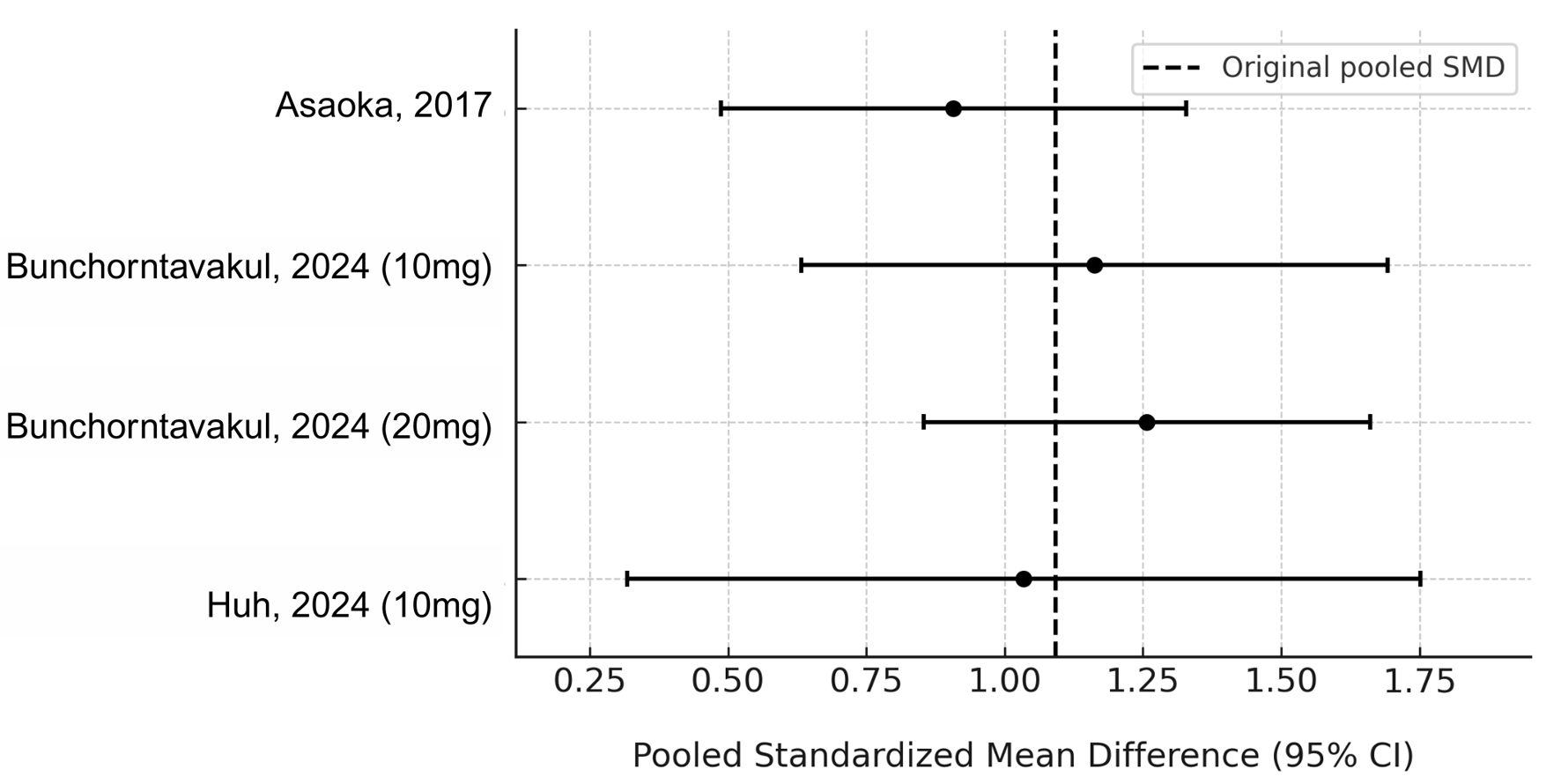 Click for large image | Figure 3. Leave-one-out sensitivity analysis of pooled standardized mean difference in symptom improvement. |
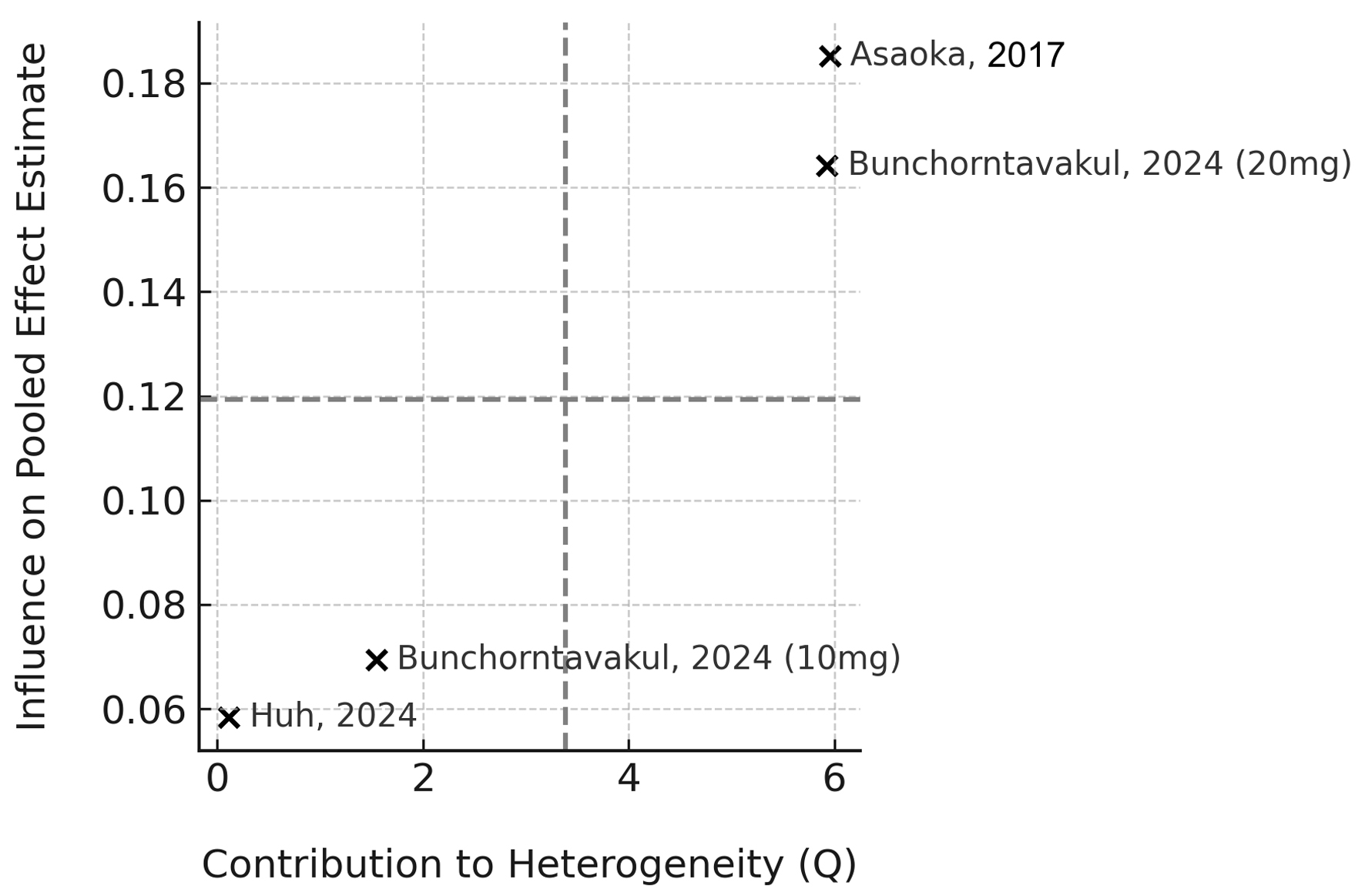 Click for large image | Figure 4. Baujat plot showing each study’s contribution to heterogeneity and influence on the pooled effect estimate. |
Narrative summary of non-pooled studies
Two studies were excluded from the meta-analysis due to incompatible outcome structures or timepoints but demonstrated symptom improvement consistent with the pooled findings. Nakamura et al (n = 42) assessed vonoprazan over a 24-week period using domain-specific GSRS sub-scores [9]. Mean reductions were observed in epigastric pain (1.39 ± 0.30), epigastric burning (-0.97 ± 0.30), and postprandial fullness (-0.90 ± 0.28), all statistically significant (P < 0.001). The study was excluded due to the extended follow-up window and non-composite scoring structure.
Shinozaki et al (n = 48) used a modified Izumo Scale to assess symptoms at 4 weeks following 10 mg vonoprazan [10]. Although mean post-treatment values were not explicitly reported, a 55% reduction from baseline (median 9.0, interquartile range (IQR) 7.0 - 12.5) was documented, suggesting meaningful clinical improvement. The study was excluded from the pooled analysis due to lack of baseline treatment values, post-treatment values, and standard deviation reporting, which precluded calculation of an SMD.
Additionally, one included study reported outcomes at both 4 and 8 weeks; only the 4-week data were used in the pooled analysis to ensure consistency in follow-up timepoints [8].
| Discussion | ▴Top |
This meta-analysis suggests that PCABs may offer clinically meaningful symptom relief in FD as reflected by a pooled SMD of 1.09 across four treatment arms. While heterogeneity remained high (I2 = 77.8%), the direction of effect was consistent, and sensitivity analyses confirmed that no single study unduly influenced the pooled estimate. Visual tools such as influence and Baujat plots further supported the stability of findings.
These results are notable given the modest and inconsistent symptom improvement often observed with PPIs in FD. The pharmacologic advantages of PCABs, particularly their rapid onset and prolonged acid suppression, may help explain their efficacy, especially in acid-sensitive subtypes such as epigastric pain syndrome [11]. Importantly, symptom improvement was observed across multiple validated scales, suggesting that the effect may be generalizable across symptom domains. However, variation in outcome instruments introduces conceptual heterogeneity and warrants cautious interpretation of effect size magnitude.
This review also underscores ongoing gaps in the evidence base. There remains a shortage of placebo-controlled or head-to-head trials, and long-term safety and efficacy data are lacking. More rigorous investigations are needed to clarify PCABs’ comparative effectiveness, optimal dosing duration, and utility across FD subtypes. While preliminary, these findings support a potential role for PCABs in the management of FD. Until confirmatory data emerge, they should be considered a promising option for patients who fail or cannot tolerate PPI therapy, with the understanding that evidence remains evolving.
There are important limitations to address in this review. First, the sample size was modest, with only four treatment arms from three studies (n = 276) being included in the meta-analysis. While all studies demonstrated directionally consistent improvement in symptoms, the small number of pooled datasets reduces statistical power and limits generalizability. Additionally, methodological heterogeneity was considerable. Differences in symptom measurement tools (NDI, modified GOSS), PCAB agent and dosing (vonoprazan 10 - 20 mg, tegoprazan 50 mg), and patient populations likely contributed to the high observed heterogeneity (I2 = 77.8%). Although use of SMDs enabled cross-study comparison, conceptual heterogeneity remains an interpretive challenge.
While both vonoprazan and tegoprazan are PCABs, their pharmacokinetic properties and acid suppression profiles differ. Vonoprazan demonstrates a faster onset and more sustained acid inhibition, which recent data suggest may translate into superior clinical efficacy compared with tegoprazan [12]. However, the small number of studies available for each individual agent precluded meaningful subgroup analysis in our review. As a result, vonoprazan and tegoprazan were pooled together in the quantitative synthesis, which should be interpreted with caution. This limitation highlights the need for future head-to-head or stratified studies to clarify potential differences in efficacy between individual PCABs.
An additional consideration is the potential role of H. pylori infection in dyspeptic symptoms. As FD is defined by the absence of organic causes, including H. pylori-associated dyspepsia, active infection should not have confounded the included populations. However, most studies did not explicitly report H. pylori testing or eradication status, which limits certainty regarding the uniformity of patient selection. Future trials should clearly document H. pylori evaluation to enhance interpretability and external validity.
Conclusion
This systematic review and meta-analysis suggest that PCABs may offer short-term symptom improvement in patients with FD, with a large pooled SMD observed across four treatment arms. Despite substantial heterogeneity, the consistency in direction of effect across studies supports the potential value of further investigation.
These findings are limited by variability in outcome measures, modest sample size, and short follow-up intervals. However, the inclusion of one study demonstrating sustained improvement at 24 weeks highlights the need for longer-term, well-controlled trials. PCABs may represent a promising therapeutic option for FD, but current evidence is preliminary and further well-designed, placebo-controlled, and comparative trials are needed to establish their role.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for this study.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
HN conceptualized the project, drafted the methodology, performed the systematic literature review, processed the statistics, drafted the manuscript, and worked revisions. TK performed the systematic literature review, drafted the manuscript, and provided revisions to the final document. AV assisted in drafting the methodology, provided key clinical insight, assisted in drafting the manuscript, and helped finalize the manuscript for submission.
Data Availability
All data analyzed during this study were extracted from published articles. Additional data and materials are available from the corresponding author upon reasonable request.
Abbreviations
FD: functional dyspepsia; GERD: gastroesophageal reflux disease; GOSS: Global Overall Symptom Scale; GSRS: Gastrointestinal Symptom Rating Scale; IQR: interquartile range; NDI: Nepean Dyspepsia Index; NSAID: non-steroidal anti-inflammatory drug; PCAB: potassium-competitive acid blocker; PPI: proton pump inhibitor; SMD: standardized mean difference
| References | ▴Top |
- Ford AC, Mahadeva S, Carbone MF, Lacy BE, Talley NJ. Functional dyspepsia. Lancet. 2020;396(10263):1689-1702.
doi pubmed - Lee K, Kwon CI, Yeniova AO, Koyanagi A, Jacob L, Smith L, Lee SW, et al. Global prevalence of functional dyspepsia according to Rome criteria, 1990-2020: a systematic review and meta-analysis. Sci Rep. 2024;14(1):4172.
doi pubmed - Oshima T. Functional dyspepsia: current understanding and future perspective. Digestion. 2024;105(1):26-33.
doi pubmed - Lacy BE, Chase RC, Cangemi DJ. The treatment of functional dyspepsia: present and future. Expert Rev Gastroenterol Hepatol. 2023;17(1):9-20.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Asaoka D, Nagahara A, Hojo M, Matsumoto K, Ueyama H, Matsumoto K, Izumi K, et al. Efficacy of a potassium-competitive acid blocker for improving symptoms in patients with reflux esophagitis, non-erosive reflux disease, and functional dyspepsia. Biomed Rep. 2017;6(2):175-180.
doi pubmed - Bunchorntavakul C, Jaigla P. Efficacy of vonoprazan 10 mg and 20 mg for patients with proton pump inhibitor-refractory functional dyspepsia: a double-blinded, randomized study. JGH Open. 2024;8(12):e70082.
doi pubmed - Huh CW, Youn YH, Jung DH, Cha RR, Kim YJ, Jung K, Song KH, et al. Efficacy of tegoprazan in patients with functional dyspepsia: a prospective, multicenter, single-arm study. J Neurogastroenterol Motil. 2024;30(3):313-321.
doi pubmed - Nakamura K, Futagami S, Agawa S, Higashida S, Onda T, Kawawa R, Habiro M, et al. Long-term vonoprazan and acotiamide-refractory patients with functional dyspepsia partly exhibit pancreatic enzyme abnormalities. Cureus. 2024;16(9):e70371.
doi pubmed - Shinozaki S, Osawa H, Hayashi Y, Miura Y, Sakamoto H, Yano T, Lefor AK, et al. Vonoprazan therapy is as effective for functional dyspepsia without heartburn as acotiamide therapy. J Gastrointestin Liver Dis. 2023;32(1):23-29.
doi pubmed - Ahmed S, Asghar S, Khanzada M, Soxi F, Gundala Raja H, Gul M, Tariq MM, et al. Comparing proton pump inhibitors and emerging acid-suppressive therapies in gastroesophageal reflux disease: a systematic review. Cureus. 2025;17(5):e84311.
doi pubmed - Kanu JE, Soldera J. Treatment of Helicobacter pylori with potassium competitive acid blockers: A systematic review and meta-analysis. World J Gastroenterol. 2024;30(9):1213-1223.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.