Increased Risk of Erosive Gastritis Using Oral Sulfate Tablet Bowel Preparation: A Retrospective Single-Center Cohort Study
DOI:
https://doi.org/10.14740/gr2045Keywords:
Oral sulfate tablet, Gastric ulcers, Gastric erosions, Bowel preparationAbstract
Background: In 2020, the Food and Drug Administration approved an oral sulfate tablet (OST) containing sodium sulfate, magnesium sulfate, and potassium chloride as a colonoscopy preparation that is more palatable and convenient than polyethylene glycol (PEG) with comparable efficacy. There is a precautionary warning regarding potential risk of mucosal ulcerations in patients with inflammatory bowel disease (IBD), but post-marketing reports describing gastritis and gastric ulceration have been sporadically reported, and the occurrence of gastric erosive disease in non-IBD patients has not been extensively documented. This study aimed to assess the occurrence of erosive gastritis in adult patients who received OST bowel preparation compared to patients who received PEG prior to same-day esophagogastroduodenoscopy (EGD) and colonoscopy.
Methods: A single-center, blinded, retrospective study was conducted of adults who underwent same-day EGD and colonoscopy. A total of 177 patients who received OST were matched with 219 patients who received PEG. Data collection involved a detailed review of the patients’ demographics, procedural, and pathology reports.
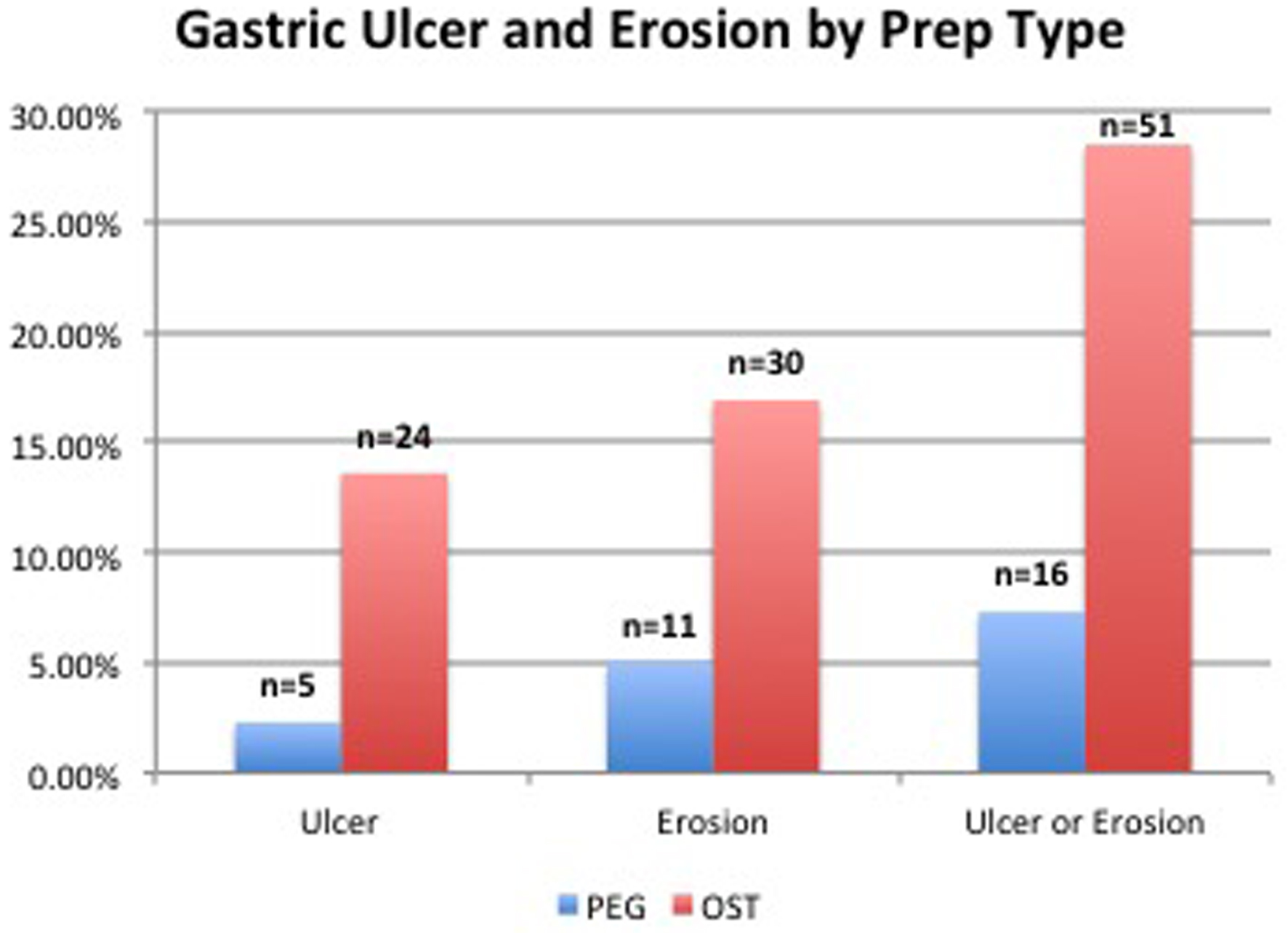
Results: The OST group demonstrated a significantly higher incidence of both gastric ulcers (13.6% vs. 2.3%, P-value < 0.001) and erosions (16.9% vs. 5.0%, P-value < 0.001) compared to the PEG group. There was no statistically significant difference between the locations of erosions and ulcers between groups.
Conclusion: The use of OST bowel preparation prior to colonoscopy is associated with increased incidence of erosive gastritis compared to PEG in patients undergoing EGD and colonoscopy on the same day at our center. Prospective, randomized studies are needed to definitively establish the risks associated with OST and to evaluate the mechanism by which it may increase the occurrence of mucosal injury.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









