Predictors of Development of Hepatocellular Carcinoma in Non-Cirrhotic Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis - A Retrospective Analysis of the National Inpatient Sample Database
DOI:
https://doi.org/10.14740/gr2053Keywords:
Liver cirrhosis, Hepatocellular carcinoma, Non-cirrhotic, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Metabolic dysfunction-associated steatotic liver disease, Metabolic dysfunction-associated steatohepatitisAbstract
Background: One-quarter of the world population is thought to have metabolic dysfunction-associated steatotic liver disease (MASLD). The incidence of metabolic dysfunction-associated steatohepatitis (MASH) and MASLD is rapidly increasing due to the ongoing global epidemic of type 2 diabetes mellitus and obesity. Hepatitis B and C have declined in incidence due to advances in prevention and treatment, yet the overall burden of hepatocellular carcinoma (HCC) continues to rise, largely driven by the growing prevalence of MASLD/MASH. MASLD/MASH is now the fastest-growing etiology of HCC in the USA, France and the UK, with an estimated annual incidence of HCC ranging from 0.5% to 2.6% in patients with MASH cirrhosis. The incidence of HCC among patients with non-cirrhotic MASLD/MASH is lower, approximately 0.1% to 1.3%. There are no screening guidelines currently for HCC in non-cirrhotic MASLD/MASH patients. Our study highlights the dire need to develop HCC predictive strategies and algorithm in this non-cirrhotic population and to move away from a cirrhotic-centered approach but rather use risk-based models. We have identified predictors of development of HCC in this patient population that can be used to develop risk-based HCC screening guidelines and models in a non-cirrhotic population with MASLD/MASH.
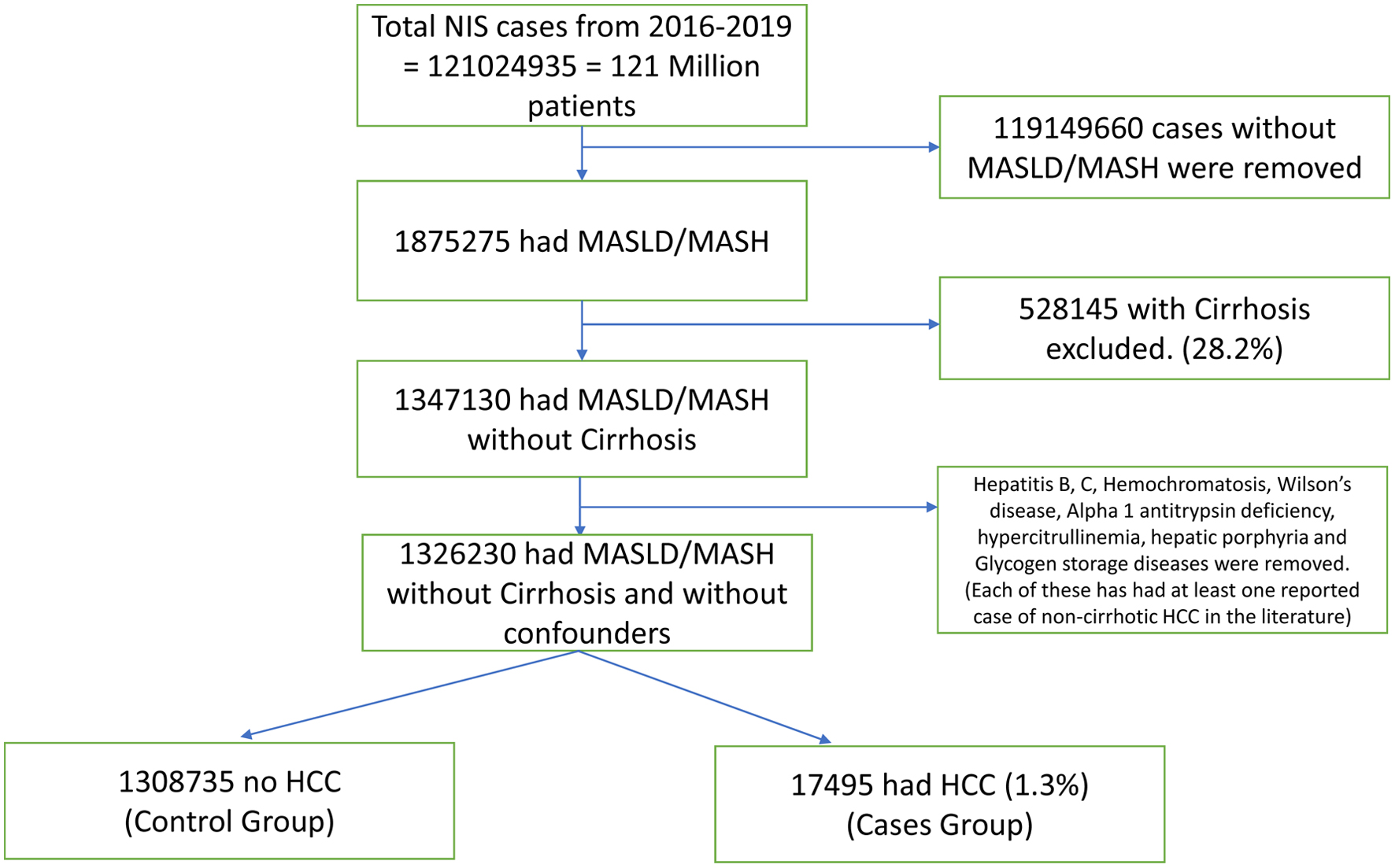
Methods: Nationwide Inpatient Sample (NIS) database from 2016 to 2019 was used in this analysis. Chi-square test and t-test and were used to establish association between two variables. The significant variables were included in the logistic regression model to identify independent association between variables.
Results: From the NIS database, 1,326,230 non-cirrhotic MASLD/MASH patients were identified. The mean age was 53.75 years; 52% were female. Older age (P < 0.0001), female gender (adjusted odds ratio (AOR) = 1.303, P < 0.001), and Asian race (AOR = 1.135, P = 0.01) were associated with increased HCC risk. Anemia, leukopenia, hyponatremia, and hypoalbuminemia were independent predictors (all P < 0.001). Benign liver lesions such as focal nodular hyperplasia (AOR = 1.269) and hemangiomas (1.475), as well as infections like cholangitis (3.093) and liver abscess (2.073), were linked to higher risk. Autoimmune diseases, including rheumatoid arthritis (0.679) and systemic lupus erythematosus (SLE, 0.456), were associated with decreased HCC risk (P < 0.001).
Conclusions: This study provides compelling evidence that HCC can develop in non-cirrhotic MASLD/MASH patients. These findings highlight an urgent need to shift from a cirrhosis-centric approach to a more comprehensive, risk-based HCC screening model - especially given that MASLD/MASH is now the most common and fastest-growing etiology of HCC around the globe. This study can potentially help develop those screening guidelines to prevent the development or early detection of HCC in non-cirrhotic MASLD/MASH patients.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









