Efficacy of Potassium-Competitive Acid Blockers in Functional Dyspepsia: A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.14740/gr2059Keywords:
Functional dyspepsia, Potassium-competitive acid blockers, Vonoprazan, Tegoprazan, Acid suppressionAbstract
Background: Functional dyspepsia (FD) is a prevalent gastrointestinal disorder characterized by upper abdominal discomfort, often refractory to standard acid suppression therapy. Potassium-competitive acid blockers (PCABs), such as vonoprazan and tegoprazan, provide rapid and sustained gastric acid inhibition and may represent a therapeutic alternative. However, their efficacy in FD remains unclear. This systematic review and meta-analysis aimed to evaluate the effect of PCAB therapy on symptom outcomes in adults with FD.
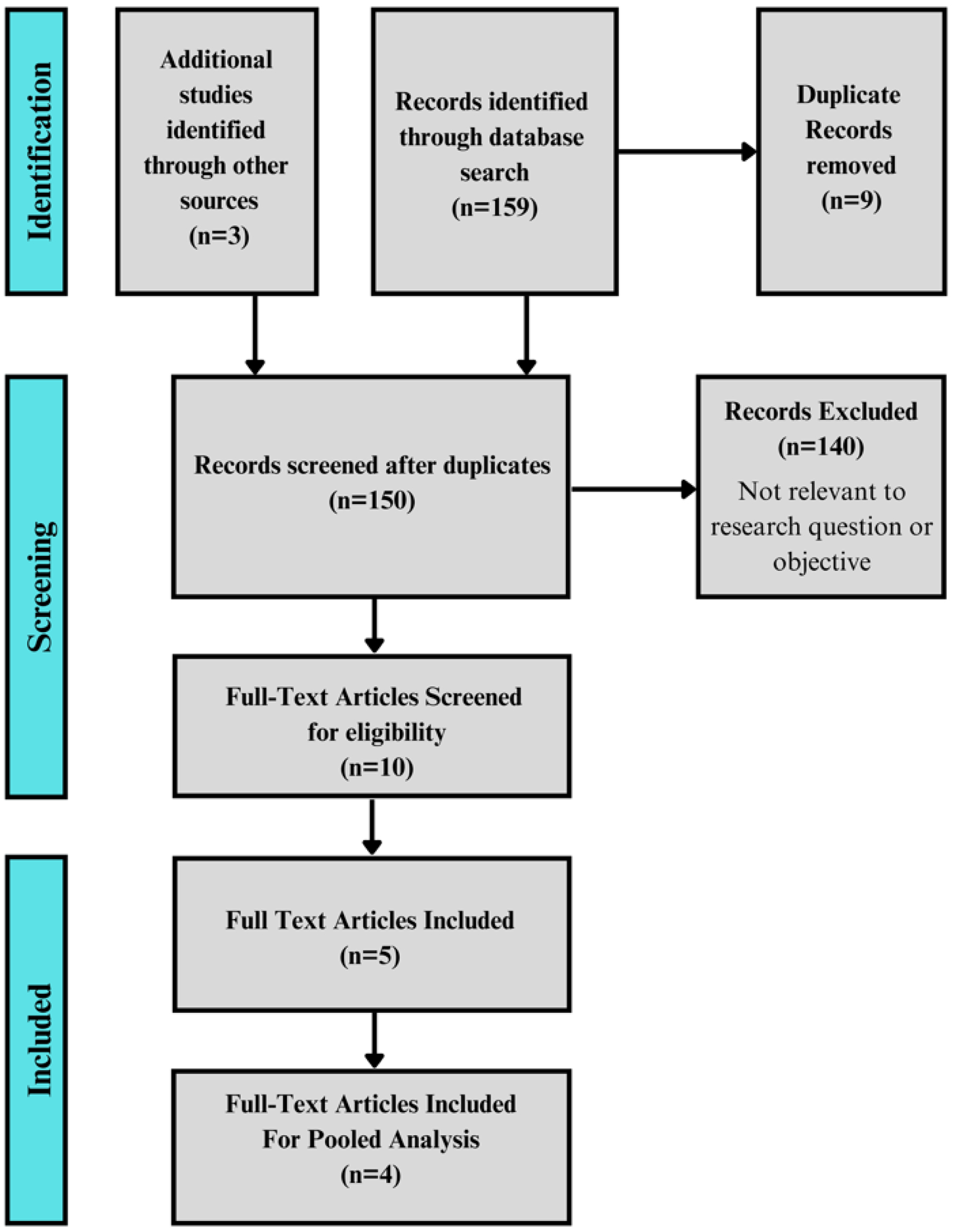
Methods: A systematic search of PubMed, EMBASE, and Cochrane CENTRAL was conducted from inception to June 13, 2025, to identify studies evaluating PCABs in adult FD patients. Eligible studies included randomized controlled trials and prospective or retrospective cohorts reporting validated symptom scores before and after PCAB therapy. Studies were included in meta-analysis if they reported 4-week outcomes with sufficient data for effect size calculation. Standardized mean differences (SMDs) were pooled using a DerSimonian-Laird random-effects model. Heterogeneity was assessed with the I2 statistic, and robustness was evaluated with leave-one-out sensitivity analysis and Baujat plots.
Results: Five studies comprising 366 patients were included. Four treatment arms from three studies (n = 276) were eligible for meta-analysis. The pooled SMD for symptom improvement at 4 weeks was 1.09 (95% confidence interval (CI): 0.67 - 1.52), indicating a moderate-to-large treatment effect in favor of PCABs. Heterogeneity was substantial (I2 = 77.8%), but sensitivity analyses showed that no single study unduly influenced results. The remaining two studies, excluded from quantitative pooling due to incompatible timepoints or outcome structures, also demonstrated statistically significant symptom improvement, supporting consistency of effect across the evidence base.
Conclusion: PCABs may offer clinically meaningful symptom relief in FD, with pooled data suggesting a moderate-to-large effect size. While heterogeneity and limited sample size temper generalizability, findings were consistent across studies and robust to sensitivity testing. PCABs show promise as a potential therapeutic option in FD, particularly in patients who do not respond to or cannot tolerate proton pump inhibitors. Further placebo-controlled trials are needed to confirm efficacy and define long-term outcomes.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









