Efficacy and Safety of Tofacitinib for Acute Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.14740/gr2086Keywords:
Ulcerative colitis, Tofacitinib, Acute severe ulcerative colitisAbstract
Background: Acute severe ulcerative colitis (ASUC) is associated with a high risk of colectomy. About 30% of patients do not respond to steroids, requiring rescue therapy. This study aims to evaluate the efficacy and safety of tofacitinib in ASUC.
Methods: MEDLINE, Embase, and Cochrane Library were systematically searched. We used random-effects model to calculate pooled proportions with 95% confidence intervals (CIs). For outcomes with ≥ 2 comparative studies, we conducted pairwise meta-analyses and calculated pooled odds ratios (ORs) with 95% CIs.
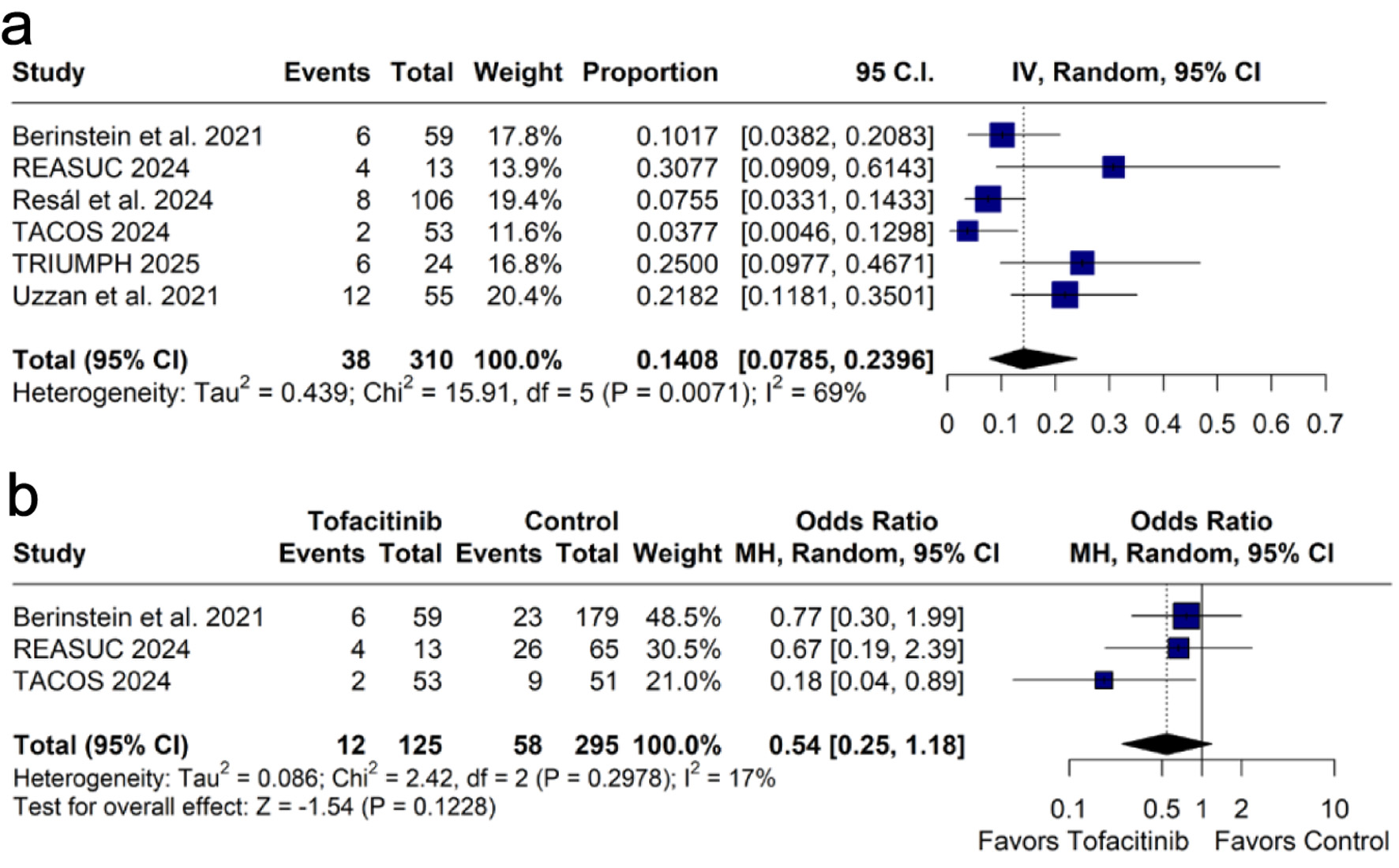
Results: We included six studies. Tofacitinib had a 90-day colectomy rate of 15.1% (95% CI: 8.5-25.3%). The clinical response rate at weeks 12 - 14 was 45.4% (95% CI: 32.6-58.9%), and at week 52 was 30.0% (95% CI: 17.4-46.5%). The clinical remission rate at weeks 12 - 14 was 38.1% (95% CI: 28.7-48.5%), and at week 52 was 27.1% (95% CI: 15.2-43.5%). Steroid-free clinical remission rate was 28.6% (95% CI: 22.2-36.1%) at weeks 12 - 14 and 33.1% (95% CI: 25.6-41.6%) at week 52. The most common adverse events were Clostridioides difficile infection, nausea, cardiovascular events, arthralgia or myalgia, herpes zoster infection, venous thromboembolism, and pneumonia. There was no significant difference in 90-day colectomy rate between tofacitinib and control (OR: 0.52; 95% CI: 0.25 - 1.08; P = 0.08).
Conclusion: Tofacitinib demonstrated high clinical response and remission rates, and low adverse events rate. Additionally, there was a trend toward a lower 90-day colectomy rate compared to controls.
Downloads
- HTML
- Supplementary Table 1
- Supplementary Table 2
- Supplementary Table 3
- Supplementary Table 4
- Supplementary Figure 1
- Supplementary Figure 2
- Supplementary Figure 3
- Supplementary Figure 4
- Supplementary Figure 5
- Supplementary Figure 6
- Supplementary Figure 7
- Supplementary Figure 8
- Supplementary Figure 9
- Supplementary Figure 10
- Supplementary Figure 11
- Supplementary Figure 12
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









