| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://gr.elmerpub.com |
Original Article
Volume 18, Number 3, June 2025, pages 139-148
Comparison of Prophylactic Transcatheter Arterial Embolization and Standard Therapy in High-Risk Non-Variceal Upper Gastrointestinal Bleeding: A Meta-Analysis
Shahryar Khana, g, Mashal Alam Khanb, Ahmed Khan Jadoonc, Ahmad Khanb, Danish Ali Khanb, Mehwish Goharb, Muhammad Shafiqa, Muhammad Waqar Elahid, Muhammad Shahzile, Tuba Esfandyarif
aDepartment of Medicine, University of Kansas Medical Center, Kansas City, KS, USA
bDepartment of Medicine, Khyber Medical University, Peshawar, Pakistan
cDepartment of Radiology, Aga Khan University Hospital, Karachi, Pakistan
dDepartment of Medicine, West Virginia University School of Medicine, Morgantown, WV, USA
eDepartment of Internal Medicine at Penn State Health Milton S. Hershey Medical Center, Pennsylvania State University, Hershey, PA, USA
fDepartment of Gastroenterology, University of Kansas Medical Center, Kansas City, KS, USA
gCorresponding Author: Shahryar Khan, Department of Medicine, University of Kansas Medical Center, Kansas City, KS, USA
Manuscript submitted April 4, 2025, accepted May 15, 2025, published online June 4, 2025
Short title: Prophylactic Embolization for High-Risk NVUGIB
doi: https://doi.org/10.14740/gr2041
| Abstract | ▴Top |
Background: Rebleeding is a major challenge and a serious complication of non-variceal upper gastrointestinal bleeding (NVUGIB). Prophylactic transcatheter arterial embolization (P-TAE) has emerged as a potential management strategy for high-risk cases. This study aimed to evaluate the efficacy and safety of P-TAE compared with no embolization (NE) in the absence of angiographic evidence of bleeding or therapeutic arterial embolization (TAE).
Methods: The study systematically searched Medline and Embase databases from inception until November 15, 2024. The primary outcome was the overall rebleeding rate, while secondary outcomes included mortality, need for additional interventions, transfusion requirements, hospital/intensive care unit (ICU) stay, and procedure-related adverse events.
Results: The meta-analysis included 10 studies with a total population of 1,253 patients. Compared to NE, the pooled data indicated that P-TAE was not associated with significantly reduced rates of rebleeding (odds ratio (OR): 0.69, 95% confidence interval (CI): 0.39 - 1.22, P = 0.20) or all-cause mortality (OR: 0.70, 95% CI: 0.40 - 1.23). Although P-TAE trended towards lower rates of repeat interventions, blood transfusions, and shorter hospital stays, these differences were not statistically significant. Conversely, P-TAE and TAE had similar rates of rebleeding (OR: 1.08, 95% CI: 0.70 - 1.68, P = 0.05) and all-cause mortality (OR: 0.72, 95% CI: 0.34 - 1.51, P = 0.39). The analysis found no significant differences in adverse events or the need for repeat procedures between the two embolization approaches.
Conclusion: This review suggests that P-TAE may not significantly reduce rebleeding or mortality compared with standard therapy for high-risk NVUGIB. However, the current findings remain inconclusive, and further comprehensive research with larger sample sizes is required to conclusively substantiate these observations.
Keywords: Prophylactic transcatheter arterial embolization; Non-variceal upper gastrointestinal bleeding; Rebleeding; Mortality; Endoscopic hemostasis; High-risk patients; Meta-analysis/systematic review; Adverse events/complications
| Introduction | ▴Top |
Upper gastrointestinal hemorrhage is a severe medical emergency that frequently requires hospitalization, with incidence rates ranging from 37 to 172 cases per 100,000 individuals [1, 2]. This condition significantly contributes to morbidity and mortality, with reported mortality rates ranging between 2% and 10% [2]. In the United States, the estimated in-hospital mortality from this condition is approximately 78 cases per 100,000 individuals, leading to over 300,000 hospital admissions and approximately 30,000 deaths annually [3]. The majority of upper gastrointestinal bleeding cases are attributed to non-variceal etiologies, such as peptic ulcers, Mallory-Weiss tears, and erosive gastritis. The management of non-variceal upper gastrointestinal bleeding (NVUGIB) is well-established in clinical guidelines and supported by a strong evidence base [4]. Following successful endoscopic hemostasis in patients with NVUGIB, clinical guidelines recommend standard medical management, including proton pump inhibitors and, when necessary, treatment for Helicobacter pylori infection [4]. If bleeding recurs, therapeutic arterial embolization (TAE) or surgery may be considered, with TAE often favored owing to its safety profile [4, 5]. Continuous risk assessment is needed to identify patients at a higher risk of rebleeding, allowing for focused monitoring and treatment.
Although endoscopic intervention is the foundation of management, a significant proportion of patients still experience refractory or recurrent bleeding despite endoscopic hemostasis [5]. Despite advancements in healthcare, the challenge of rebleeding in high-risk patients with NVUGIB remains a significant concern. However, approximately 12-25% of patients develop clinically significant rebleeding, which increases mortality [6, 7]. Over the past two decades, TAE has emerged as a potential first-line intervention for managing upper gastrointestinal bleeding refractory to endoscopic hemostasis. TAE has demonstrated beneficial outcomes and has been proposed as a less risky alternative to surgery, particularly in patients who are unable to undergo surgical procedures. Furthermore, TAE has shown a high technical success rate, a low complication rate, and does not prolong hospital stay [8].
Due to the limitations of angiographic imaging in identifying the source of bleeding, as evidenced by contrast extravasation, pseudoaneurysm, or arterial spasm, the approach of endoscopy-directed embolization, also known as “prophylactic embolization,” has emerged as a potential strategy [9]. When considering prophylactic transcatheter arterial embolization (P-TAE), the angiographer utilizes endoscopic findings to target the vascular territory that may harbor the injured vessel and embolizes it, even in the absence of active extravasation on the angiogram [10]. Several studies have evaluated the clinical outcomes of this P-TAE technique in comparison with standard therapy, yielding variable results. The potential benefits of employing P-TAE following endoscopic hemostasis have not been adequately evaluated. The optimal strategy to prevent further bleeding in this patient population has yet to be clearly defined. This systematic review and meta-analysis aimed to rigorously assess the comparative efficacy and safety of P-TAE versus standard therapy in managing high-risk NVUGIB. By synthesizing evidence from multiple studies, this analysis will provide clinicians with valuable insights to guide the optimal management of this challenging patient population.
| Materials and Methods | ▴Top |
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [11]. Given the absence of patient-specific data collected, ethical approval was not required for this study.
Inclusion and exclusion criteria
Studies meeting the following criteria were included: 1) Patients were adults (age ≥ 18 years) with proven high-risk NVUGIB confirmed by endoscopy or computed tomography angiography (population). High-risk NVUGIB was defined as bleeding from ulcers classified as Forrest I-IIb and patients exhibiting clinical evidence of persistent gastrointestinal bleeding. 2) Patients without angiographic evidence of arterial bleeding underwent P-TAE at a high risk of recurrent bleeding (intervention). 3) Patients without angiographic evidence of arterial bleeding who did not undergo embolization, or patients with evidence of arterial bleeding who received TAE (control). 4) The outcomes included any of the following: a) overall rebleeding (such as in-hospital and 30-day); b) all-cause mortality rates; c) need for additional interventions, such as repeat endoscopy, salvage embolization, and surgery; d) the length of hospital and intensive care unit (ICU) stay; e) requirement for blood transfusions; and f) procedure-related adverse events.
Studies were excluded if they lacked a comparison group; were published in a language other than English; were case reports, editorials, or letters; or did not assess relevant outcome measures. Both observational and interventional studies were included, whereas case reports, case series with fewer than 10 patients, editorials, guidelines, abstracts, and review articles were excluded.
Search strategy and study selection
We designed a comprehensive search strategy and implemented it across EMBASE and PubMed, covering all publications from inception to November 15, 2024. This strategy combines free-text and MeSH terms, incorporating synonyms and spelling variations. The full search strategy is available in Supplementary Material 1 (gr.elmerpub.com). We also manually searched the reference lists of all identified trials, guidelines, and reviews on the topic. All citations were imported into Covidence. Two reviewers (MK and AA) independently screened titles/abstracts and full-text articles, and discrepancies were resolved by a third reviewer (SK). Data extraction was performed in duplicate by MK and AA using standardized forms, including study identification (e.g., authorship, publication year, country of origin), study design and risk of bias assessment, patient demographics (e.g., age, sex, comorbidities), intervention and comparator descriptions, and outcomes. Relevant subgroup data where available were also collected.
Data synthesis and analysis
The analysis utilized odds ratios (ORs) with 95% confidence intervals (CIs) to evaluate categorical outcomes, and standardized mean differences (SMDs) with 95% CIs for continuous outcomes. Statistical significance was defined as an alpha value of ≤ 0.05. Additionally, a random-effects model with restricted maximum likelihood estimation was used to address the observed heterogeneity across the included studies. Furthermore, Wald-type CIs were calculated based on the pooled effect size and its associated standard error, to quantify the uncertainty surrounding the summary statistics. The degree of heterogeneity was evaluated using the I2 statistics, which provides a quantitative measure of the inconsistency across study results. The I2 values were interpreted as follows: 0-30% indicated low heterogeneity, 30-60% moderate heterogeneity, 50-90% substantial heterogeneity, and 75-100% considerable heterogeneity, with a P-value < 0.1 being considered statistically significant recommended by the Cochrane Handbook. Publication bias was evaluated by visual inspection of the funnel plots. Sensitivity analyses were conducted by removing each study individually and including only those studies without a high risk of bias. Finally, we assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation framework. All statistical analyses were performed using RevMan software. Finally, the risk of bias of the included studies was assessed using the ROBINS-1 tool. Two reviewers (AA and MK) independently assessed the risk of bias in the included studies, and a third reviewer (SK) resolved any discrepancies.
| Results | ▴Top |
The study selection process adhered to the PRISMA guidelines, as outlined in Supplementary Material 2 (gr.elmerpub.com). A comprehensive literature search yielded 267 and 302 citations from PubMed and Embase, respectively. After removing duplicates, 435 articles were screened, and ultimately, 10 full-text studies [12-21] were included in the analysis, with a total population of 1,253 patients. The study population comprised 37% of patients who underwent P-TAE, 53.7% who did not undergo embolization, and 9.3% who underwent TAE. Table 1 summarizes the characteristics of the study population. The studies varied in design and originated from different countries, spanning from 2009 to 2019. Each study employed a slightly different definition of “high-risk gastrointestinal bleeding,” ranging from initial endoscopy failure to arrest bleeding, to specific Forrest classifications and Rockall scores. Five studies reported the Forrest classification, with a total of 83 (15%) patients classified as Forrest Ia, 163 (29.2%) as Ib, 247 (45.6%) as IIa, and 64 (10.2%) as IIb. The quality of the studies was assessed and summarized in Supplementary Material 3 (gr.elmerpub.com), while the overall certainty of the evidence was evaluated using the GRADE approach and presented in Supplementary Material 4 (gr.elmerpub.com).
 Click to view | Table 1. Characteristics of Included Studies |
Overall rebleeding rate
Seven studies examined the comparative effectiveness of P-TAE versus no embolization (NE) in managing high-risk NVUGIB [14-18, 20, 21]. The pooled results indicated that the utilization of P-TAE did not demonstrate a significantly lower overall incidence of rebleeding than the group that did not undergo embolization (OR: 0.69, 95% CI: 0.39 - 1.22, P = 0.20, I2 = 27%, Fig. 1). Additionally, when sensitivity analyses were restricted to randomized controlled trials, the benefits of P-TAE in preventing rebleeding were consistently not found to be superior to the lack of embolization (OR: 0.58, 95% CI: 0.18 - 1.83, P = 0.35, I2 = 45%, Fig. 2). However, when seven studies [12-15, 17-19] directly compared P-TAE with TAE, no notable difference was found between these two interventional approaches (OR: 1.08, 95% CI: 0.70 - 1.68, P = 0.05, I2 = 0%, Fig. 3). Lau et al found that P-TAE was effective in reducing the incidence of recurrent bleeding specifically among patients with ulcers ≥ 15 mm in size (hazard ratio: 5.56, 95% CI: 1.24 - 24.87, P = 0.018).
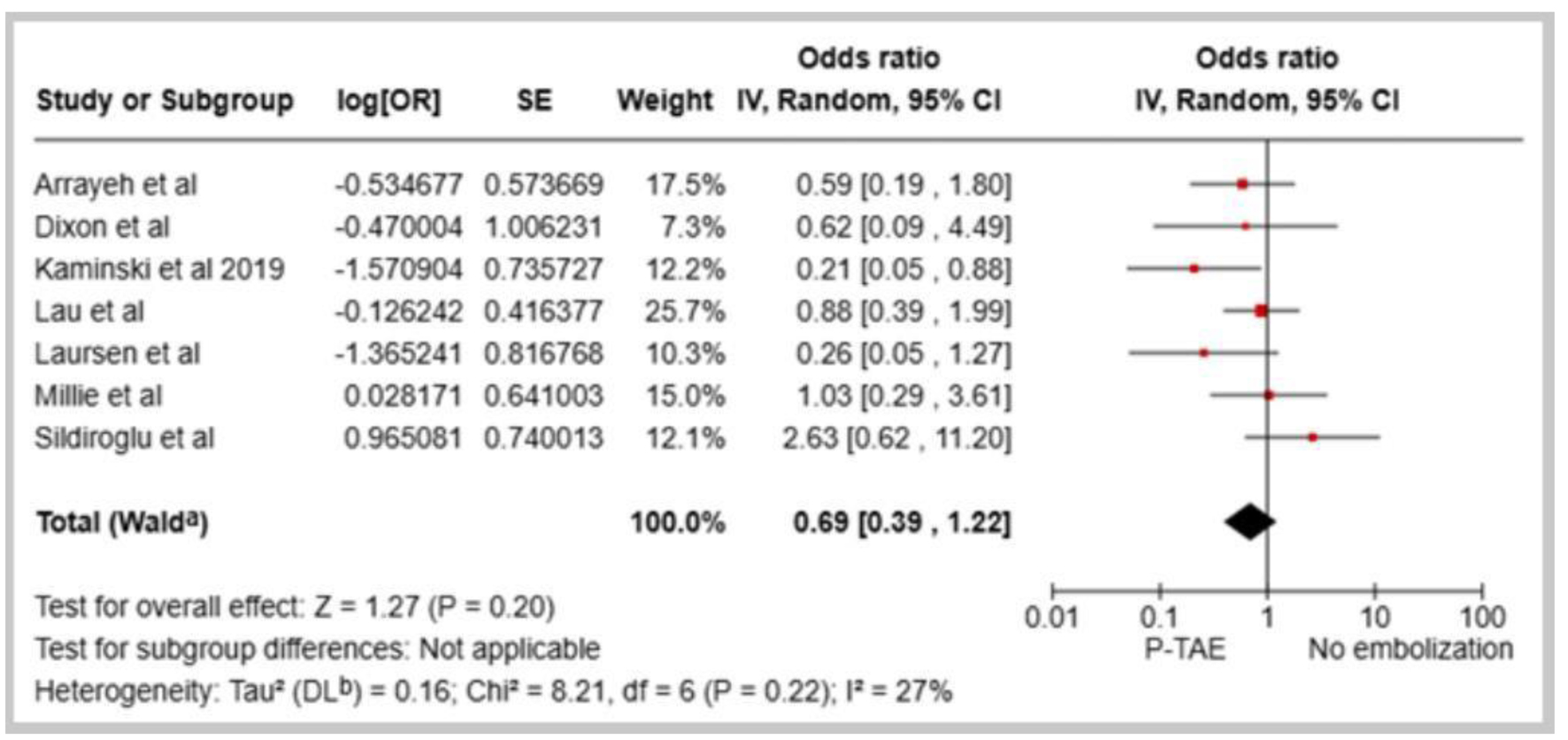 Click for large image | Figure 1. Forest plot of overall rebleeding rate in comparison between prophylactic transcatheter arterial embolization (P-TAE) and no embolization. |
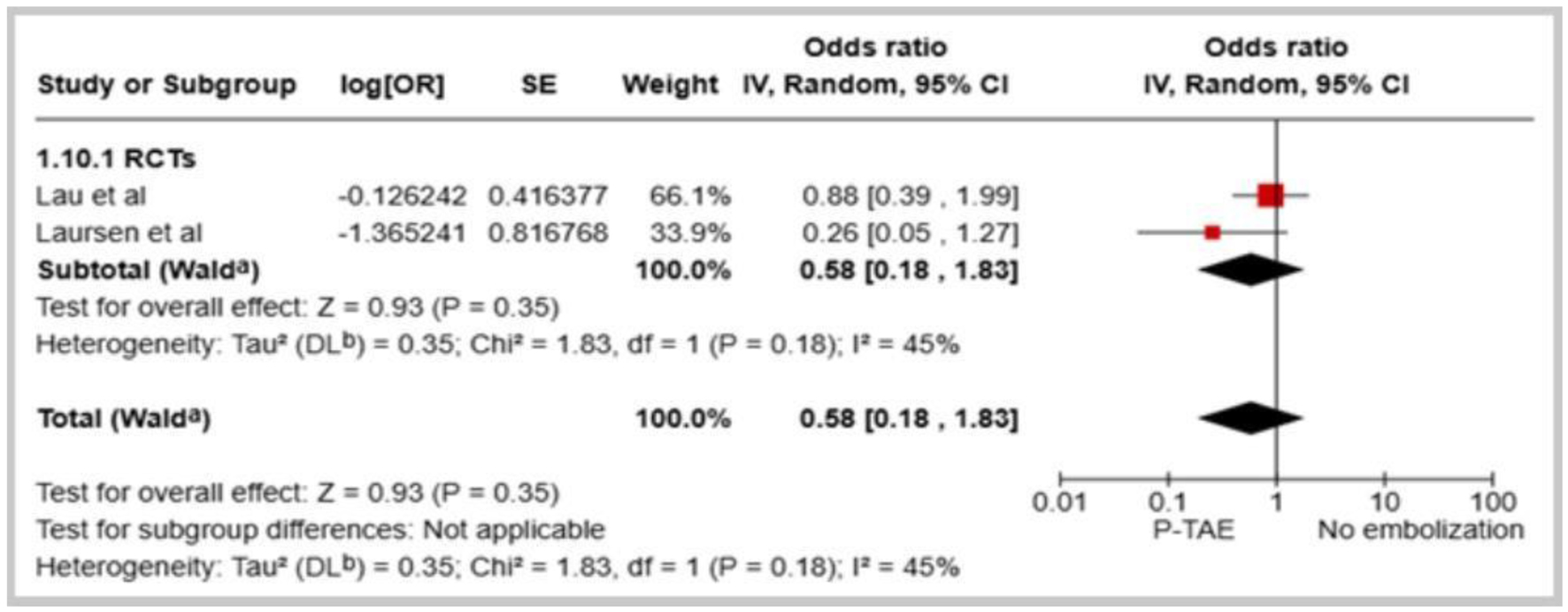 Click for large image | Figure 2. Forest plot of overall rebleeding rate in comparison between prophylactic transcatheter arterial embolization (P-TAE) and no embolization (only RCTs). |
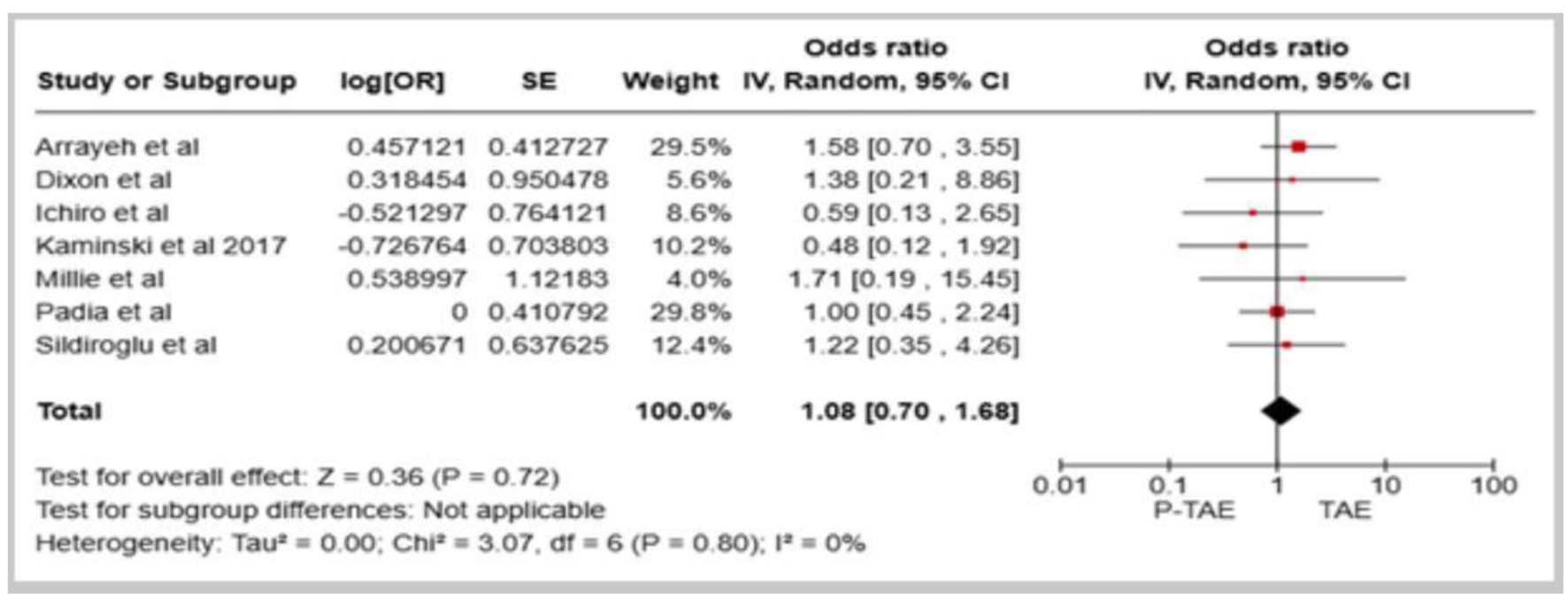 Click for large image | Figure 3. Forest plot of overall rebleeding rate in comparison between prophylactic transcatheter arterial embolization (P-TAE) and therapeutic arterial embolization (TAE). |
All-cause mortality rate and procedure-related adverse events
The meta-analysis of the eight included studies did not demonstrate a statistically significant decline in the all-cause mortality rate with the utilization of P-TAE compared with the NE group (OR: 0.70, 95% CI: 0.40 - 1.23, I2 = 0%, Fig. 4). However, two studies [16, 18] that specifically investigated 30-day mortality associated with rebleeding did not reveal a notable difference between the two interventions (OR: 1.45, 95% CI: 0.23 - 9.10, I2 = 0%, Fig. 5). Additionally, in the seven studies that directly compared P-TAE and TAE, there were no notable differences in all-cause mortality (OR: 0.72, 95% CI: 0.34 - 1.51, P = 0.39, I2 = 32%) and mortality related to rebleeding (OR: 0.75, 95% CI: 0.14 - 3.95, P = 0.73, I2 = 56%). The analysis did not detect significant differences in the incidence of procedure-related adverse events between the P-TAE and TAE (OR: 0.86, 95% CI: 0.22 - 3.45, P = 0.84, I2 = 0%, Fig. 6). Nonetheless, some rare complications associated with embolization procedures have been reported, such as access site hematoma, duodenal stenosis, pancreatitis, and coil dislocation. Notably, in a single case, a coil was mispositioned during the embolization procedure, migrating into the hepatic artery, which ultimately resulted in acute liver failure and the patient’s death [18].
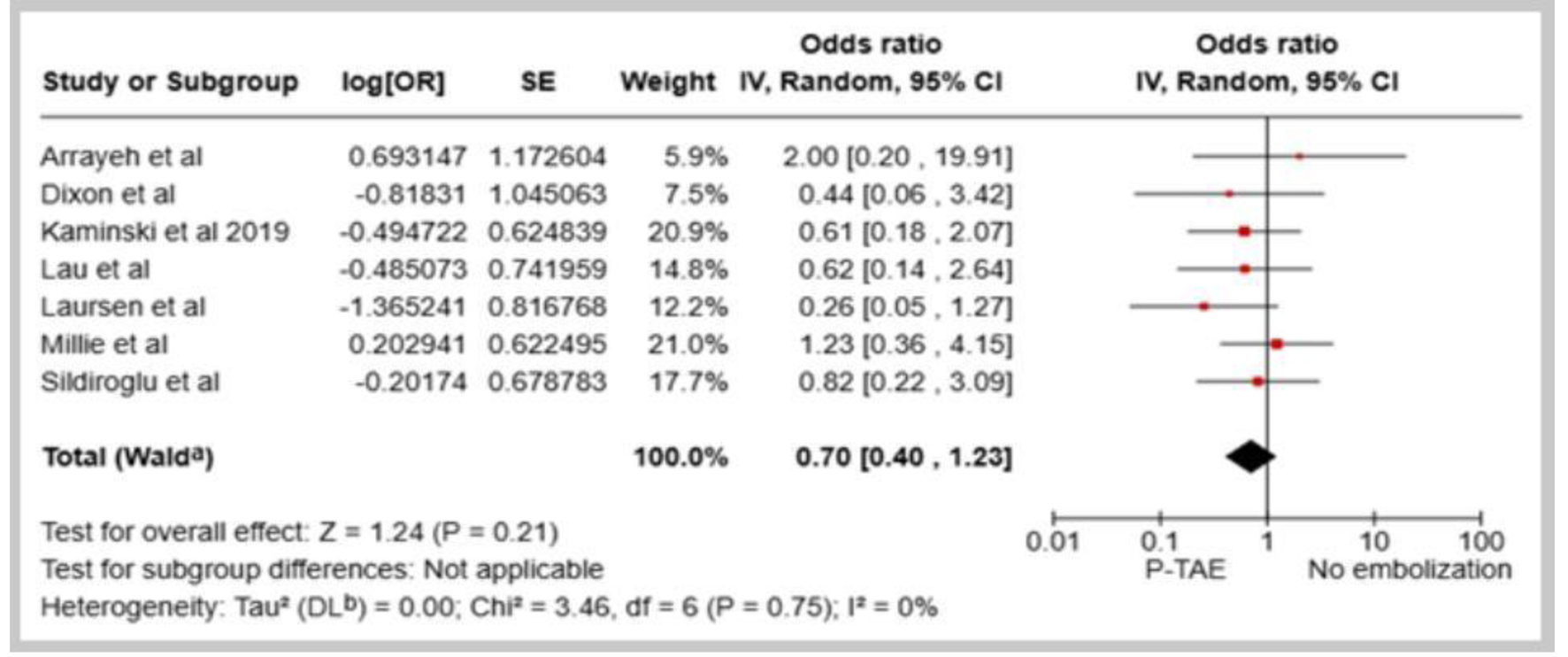 Click for large image | Figure 4. Forest plot of all-cause mortality in comparison between prophylactic transcatheter arterial embolization (P-TAE) and no embolization. |
 Click for large image | Figure 5. Forest plot of all-cause mortality in comparison between prophylactic transcatheter arterial embolization (P-TAE) and therapeutic arterial embolization (TAE). |
 Click for large image | Figure 6. Forest plot of procedure-related adverse events in comparison between prophylactic transcatheter arterial embolization (P-TAE) and therapeutic arterial embolization (TAE). |
Need for additional procedures and blood transfusions
The available evidence suggests a trend towards lower rates of reinterventions following P-TAE compared to NE, but this difference did not reach statistical significance, such as repeat endoscopy (OR: 0.57, 95% CI: 0.26 - 1.23, P = 0.15, I2 = 0%), salvage TAE (OR: 0.33, 95% CI: 0.08 - 1.31, P = 0.13, I2 = 0%), and surgical interventions (OR: 0.68, 95% CI: 0.33 - 1.40, P = 0.29, I2 = 0%). Moreover, when directly compared with TAE, P-TAE was not associated with significant differences in the rates of repeat endoscopy (OR: 1.10, 95% CI: 0.52 - 2.34, P = 0.80, I2 = 0%), salvage TAE (OR: 0.49, 95% CI: 0.17 - 1.45, P = 0.20, I2 = 15%), and surgical interventions (OR: 0.50, 95% CI: 0.19 - 1.34, P = 0.17, I2 = 32%). The analysis found no statistically significant differences in the number of blood transfusions received between the P-TAE group and the group that did not undergo embolization (standard mean difference (SMD): 0.51, 95% CI: -0.11 to 1.13, P = 0.11, I2 = 93%). Furthermore, no significant differences were observed in the blood transfusion needs when directly comparing the P-TAE and TAE approaches (SMD: -0.19, 95% CI: -0.63 to 0.24, P = 0.38, I2 = 26%).
Length of hospital and ICU stay
The meta-analysis did not find statistically significant differences in the mean hospital length of stay (SMD: -0.0, 95% CI: -0.17 to 0.17, P = 0.98, I2 = 0%) and ICU stay (SMD: -0.07, 95% CI: -0.25 to 0.12, P = 0.48, I2 = 0%) between the P-TAE group and the NE groups. Similarly, when directly comparing P-TAE and TAE interventions, no notable differences were observed in mean hospital length of stay (SMD: -0.25, 95% CI: -0.97 to 0.46, P = 0.49, I2 = 80%), and one study reported a comparable median ICU stay of 3 days between the two groups [20].
| Discussion | ▴Top |
The management of high-risk NVUGIB remains a significant challenge, despite the well-recognized role of endoscopic hemostasis. Recurrent bleeding is a serious complication associated with considerable mortality risk, particularly in an aging population with more comorbidities, where surgical intervention carries high-risk mortality rates ranging from 18% to 40% [22]. Several options are recommended, including emergent repeated endoscopy, TAE, and surgical intervention. P-TAE represents an additional strategy for decreasing the rebleeding rate after initial endoscopic hemostasis. However, the precise criteria for selecting the appropriate indications and the strong evidence supporting the rationale for employing this hemostatic approach remain insufficient [23]. In this study, we investigated the role of P-TAE in preventing rebleeding in high-risk NVUGIB patients, compared to those without angiographic evidence of arterial bleeding who did not undergo embolization, or those with evidence of arterial bleeding who received TAE. Previous systematic reviews have investigated the role of P-TAE in high-risk bleeding ulcer patients [8, 24, 25]. However, these reviews were limited to a comparison of P-TAE with the absence of embolization. In contrast to prior meta-analyses, this study provides a more thorough evaluation of P-TAE by comparing it with both the absence of embolization and TAE. This allowed for a comprehensive evaluation of the benefits of P-TAE across diverse clinical settings. Additionally, this meta-analysis assessed multiple clinically relevant outcomes, such as rebleeding, mortality, surgical intervention, hospital stay, and blood transfusions, and offered a detailed understanding of TAE’s role in managing high-risk NVUGIB. In contrast, the findings of this analysis diverged from those reported by Boros et al, who observed a significant reduction in rebleeding risk and overall mortality with the use of P-TAE compared to NE [24]. Nonetheless, this purported benefit was not upheld in sensitivity analyses restricted to randomized controlled trials.
The meta-analysis revealed a trend toward reduced rebleeding and all-cause mortality rates with P-TAE compared to instances where embolization was not performed. However, these differences were not statistically significant. This observation remained consistent even when the analysis was limited to randomized controlled trials, indicating that P-TAE is not superior to the standard treatment in preventing rebleeding. Consequently, available data suggest that P-TAE does not provide a significant survival advantage over standard treatment strategies for this patient population. A study by Lau et al indicated that the adjunctive use of angiographic embolization led to a notable decrease in the risk of recurrent bleeding among patients with upper gastrointestinal ulcers measuring 15 mm or larger [20]. Larger ulcers present an increased surface area for potential rebleeding and may be indicative of a more severe underlying pathology, thereby posing greater challenges for management with standard endoscopic therapies alone. Although P-TAE may be efficacious in mitigating recurrent bleeding in high-risk patients with larger ulcers, aggregate evidence does not definitively establish a clear benefit of P-TAE over alternative management strategies. In cases involving substantial gastroduodenal ulcers, angiographic embolization may warrant consideration; however, the presence of a visible vessel can signify a sentinel clot or thrombus [20]. Currently, there is no dependable endoscopic technique for accurately assessing the size of the bleeding artery. A randomized trial demonstrated that Doppler-signal-guided endoscopic treatment resulted in decreased rebleeding rates. Endoscopic ultrasonography-guided injection therapy has been used for refractory bleeding lesions [26]. While endoscopic angiotherapy is progressing, percutaneous angiography remains the most reliable method for visualizing and characterizing the bleeding artery. Notably, no significant differences were observed in the overall rebleeding and mortality rates between P-TAE and TAE. The most frequent causes of persistent bleeding following embolization are either failure to embolize the actual culprit vessel or inadequate reduction of perfusion pressure through embolization of the major trunk from which it originates [15].
The incidence of procedure-related adverse events was generally low and comparable between the P-TAE and TAE groups. Furthermore, the analysis did not find significant differences between the P-TAE and comparator groups in the need for additional procedures, blood transfusion requirements, or the length of hospital and ICU stay. Although TAE can be effective for managing NVUGIB, it has several disadvantages. A primary concern is the risk of non-target embolization, potentially leading to bowel ischemia or, in rare instances, more severe complications, such as acute liver failure and death [18]. Definitive hemostasis is not always achieved with TAE and rebleeding can occur [27]. TAE’s efficacy may be compromised if the bleeding is intermittent during angiography. As with any angiographic procedure, risks associated with arterial access, including hematoma, pseudoaneurysm, or infection, are present. Coagulopathy, sepsis, and renal insufficiency may increase the risk of complications associated with TAE [15]. Furthermore, TAE requires specialized expertise and equipment, which potentially limits its availability and can be a costly procedure.
Identifying and assessing risk factors is crucial for accurately predicting patients at high risk of rebleeding, which is essential for optimizing the management of NVUGIB. Several studies have utilized the Forrest classification, Rockall score, or a combination of these to identify high-risk patients eligible for P-TAE. The Forrest classification, with its detailed categorization of ulcer characteristics (e.g., active spurting, oozing, visible vessels), helps identify patients at an immediate high risk of rebleeding, guiding decisions on early intervention. Similarly, the Rockall score, which incorporates clinical and endoscopic factors, provides a comprehensive risk assessment that influences the threshold for considering P-TAE. Zetner et al suggested that individuals with high Rockall scores exhibited an elevated risk of 30-day mortality (OR: 2.58, P = 0.01) [27]. Arrayeh et al found no significant improvement in rebleeding or survival in patients with gastric hemorrhage who underwent empiric embolization. However, those who underwent embolization with documented angiographic abnormalities exhibited a trend towards better 30-day primary hemostasis. For patients with duodenal bleeding, a similar trend of improved 30-day primary hemostasis was observed, regardless of the presence or absence of an angiographic abnormality [14]. Arrayeh et al also reported that patients with duodenal bleeding from a mass lesion showed a higher rate of primary hemostasis 30 days after angiography than those with non-mass sources of duodenal bleeding. However, this distinction was not observed between the mass and non-mass lesions in the context of gastric bleeding. The presence or absence of angiographic abnormalities further influences outcomes, potentially predicting hemostasis in upper non-variceal bleeding [12, 20]. The visualization of active bleeding, pseudoaneurysms, or arteriovenous malformations during angiography allows for targeted embolization of the specific bleeding vessel, potentially improving hemostasis rates and reducing the need for further intervention. Conversely, the absence of identifiable angiographic abnormalities suggests a lower likelihood of benefit from P-TAE [14].
Several limitations should be considered when interpreting the findings of this meta-analysis. The incorporation of non-randomized controlled trials can introduce bias due to variations in patient selection, potentially distorting observed outcomes, and hindering an accurate assessment of the true effect of P-TAE. Furthermore, variations in treatment protocols, such as differences in the timing of endoscopic intervention, selection of endoscopic hemostasis techniques, or administration of adjunctive medical therapies, may contribute to inter-study heterogeneity. Moreover, variations in institutional practices across studies can introduce additional confounding variables, including differences in resource availability, provider expertise, and the implementation of clinical practice guidelines, further complicating the interpretation of the pooled results. The limited number and size of the included randomized controlled trials restrict the overall strength of the evidence and the ability to draw definitive conclusions. The duration of follow-up varied across studies, which could have affected the assessment of long-term outcomes. Furthermore, the observed heterogeneity in certain outcomes, such as the length of hospital stay, hinders interpretation, as it suggests variability in clinical practices and reporting standards. This heterogeneity could potentially affect the robustness and generalizability of the findings, which the authors sought to address through sensitivity analyses and subgroup evaluations. Despite some variability in the specific criteria used to define patients at high-risk for rebleeding across the included studies, the study populations collectively leveraged a common approach, utilizing clinical and/or endoscopic risk factors to identify individuals with an elevated risk of rebleeding.
In clinical practice, the evidence from this meta-analysis indicates that P-TAE may not yield a significant reduction in rebleeding rates or mortality in high-risk NVUGIB when compared with standard therapy or TAE alone. Considering these findings, a judicious approach for the application of P-TAE is warranted, necessitating a nuanced, multidisciplinary approach involving collaboration among specialists in endoscopy, interventional radiology, and surgery. Meticulous patient selection is of paramount importance, with careful attention directed toward factors such as ulcer size, presence of angiographic abnormalities, and the patient’s overall comorbidity profile. A comprehensive evaluation of the potential benefits of P-TAE relative to the risks of potential complications, including but not limited to non-target embolization, access site complications, and contrast-induced nephropathy, is essential in the decision-making process. Future research should concentrate on large, multicenter, randomized controlled trials employing standardized protocols to refine the patient selection criteria and procedural techniques. Longitudinal studies are necessary to evaluate the sustainability of benefits, whereas cost-effectiveness analyses can inform broader clinical implementation. Advancements in imaging and embolization technologies should be explored to augment their safety and efficacy.
Conclusion
The findings of this systematic review and meta-analysis indicate that the use of P-TAE may not provide a significant advantage in reducing overall rebleeding rates or mortality compared to the absence of embolization or TAE for the management of high-risk NVUGIB. These results highlight the need for further research to determine the optimal role of P-TAE in this challenging patient populations. Specifically, future studies should focus on refining the patient selection criteria to identify those most likely to benefit from this intervention, evaluate the long-term sustainability of any potential benefits, and assess the cost-effectiveness of implementing P-TAE in clinical practice. In addition, advancements in imaging and embolization technologies should be explored to enhance the safety and efficacy of this approach.
| Supplementary Material | ▴Top |
Suppl 1. Search strategy.
Suppl 2. PRISMA flow-diagram of included studies.
Suppl 3. Risk of bias.
Suppl 4. Outcomes grading.
Acknowledgments
None to declare.
Financial Disclosure
The author reported no financial disclosure relevant to this work.
Conflict of Interest
The author reported no conflict of interest relevant to this work.
Informed Consent
Not applicable.
Author Contributions
Shahryar Khan: conceptualization (lead); writing - original draft (lead); formal analysis (lead); writing - review and editing (lead). Mashal Alam Khan: data extraction, review, and editing. Ahmed Khan Jadoon: data extraction, review, and editing. Ahmad Khan: methodology and data extraction. Mehwish Gohar: data extraction and double-checking data. Muhammad Waqar Elahi: data extraction and double-checking data. Muhammad Shahzil: data extraction, review, and editing. Muhammad Shafiq: review, and editing. Tuba Esfandyari: mentorship, conceptualization (supporting) and review.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary information files.
| References | ▴Top |
- van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22(2):209-224.
doi pubmed - Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536.
doi pubmed - Laine L, Laursen SB, Zakko L, Dalton HR, Ngu JH, Schultz M, Stanley AJ. Severity and outcomes of upper gastrointestinal bleeding with bloody vs. coffee-grounds hematemesis. Am J Gastroenterol. 2018;113(3):358-366.
doi pubmed - Mullady DK, Wang AY, Waschke KA. AGA clinical practice update on endoscopic therapies for non-variceal upper gastrointestinal bleeding: expert review. Gastroenterology. 2020;159(3):1120-1128.
doi pubmed - Jung K, Moon W. Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: An evidence-based review. World J Gastrointest Endosc. 2019;11(2):68-83.
doi pubmed - Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008;103(10):2625-2632.
doi pubmed - Wong SK, Yu LM, Lau JY, Lam YH, Chan AC, Ng EK, Sung JJ, et al. Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut. 2002;50(3):322-325.
doi pubmed - Chang JHE, Lye TJY, Zhu HZ, Syn NL, Tang SS, Gogna A, Chan WH, et al. Systematic review and meta-analysis of prophylactic transarterial embolization for high-risk bleeding peptic ulcer disease. J Vasc Interv Radiol. 2021;32(4):576-584.e575.
doi pubmed - Loffroy R, Favelier S, Pottecher P, Estivalet L, Genson PY, Gehin S, Cercueil JP, et al. Transcatheter arterial embolization for acute nonvariceal upper gastrointestinal bleeding: Indications, techniques and outcomes. Diagn Interv Imaging. 2015;96(7-8):731-744.
doi pubmed - Shin JH. Recent update of embolization of upper gastrointestinal tract bleeding. Korean J Radiol. 2012;13(Suppl 1):S31-S39.
doi pubmed - Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
doi pubmed - Padia SA, Geisinger MA, Newman JS, Pierce G, Obuchowski NA, Sands MJ. Effectiveness of coil embolization in angiographically detectable versus non-detectable sources of upper gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20(4):461-466.
doi pubmed - Ichiro I, Shushi H, Akihiko I, Yasuhiko I, Yasuyuki Y. Empiric transcatheter arterial embolization for massive bleeding from duodenal ulcers: efficacy and complications. J Vasc Interv Radiol. 2011;22(7):911-916.
doi pubmed - Arrayeh E, Fidelman N, Gordon RL, LaBerge JM, Kerlan RK, Jr., Klimov A, Bloom AI. Transcatheter arterial embolization for upper gastrointestinal nonvariceal hemorrhage: is empiric embolization warranted? Cardiovasc Intervent Radiol. 2012;35(6):1346-1354.
doi pubmed - Dixon S, Chan V, Shrivastava V, Anthony S, Uberoi R, Bratby M. Is there a role for empiric gastroduodenal artery embolization in the management of patients with active upper GI hemorrhage? Cardiovasc Intervent Radiol. 2013;36(4):970-977.
doi pubmed - Laursen SB, Hansen JM, Andersen PE, Schaffalitzky de Muckadell OB. Supplementary arteriel embolization an option in high-risk ulcer bleeding—a randomized study. Scand J Gastroenterol. 2014;49(1):75-83.
doi pubmed - Sildiroglu O, Muasher J, Arslan B, Sabri SS, Saad WE, Angle JF, Matsumoto AH, et al. Outcomes of patients with acute upper gastrointestinal nonvariceal hemorrhage referred to interventional radiology for potential embolotherapy. J Clin Gastroenterol. 2014;48(8):687-692.
doi pubmed - Mille M, Huber J, Wlasak R, Engelhardt T, Hillner Y, Kriechling H, Aschenbach R, et al. Prophylactic transcatheter arterial embolization after successful endoscopic hemostasis in the management of bleeding duodenal ulcer. J Clin Gastroenterol. 2015;49(9):738-745.
doi pubmed - Kaminskis A, Kratovska A, Ponomarjova S, Tolstova A, Mukans M, Stabina S, Gailums R, et al. Preventive transarterial embolization in upper nonvariceal gastrointestinal bleeding. World J Emerg Surg. 2017;12:3.
doi pubmed - Lau JYW, Pittayanon R, Wong KT, Pinjaroen N, Chiu PWY, Rerknimitr R, Holster IL, et al. Prophylactic angiographic embolisation after endoscopic control of bleeding to high-risk peptic ulcers: a randomised controlled trial. Gut. 2019;68(5):796-803.
doi pubmed - Kaminskis A, Ivanova P, Kratovska A, Ponomarjova S, Ptasnuka M, Demicevs J, Demiceva R, et al. Endoscopic hemostasis followed by preventive transarterial embolization in high-risk patients with bleeding peptic ulcer: 5-year experience. World J Emerg Surg. 2019;14:45.
doi pubmed - Loffroy R, Guiu B, D'Athis P, Mezzetta L, Gagnaire A, Jouve JL, Ortega-Deballon P, et al. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7(5):515-523.
doi pubmed - Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116(5):899-917.
doi pubmed - Boros E, Sipos Z, Hegyi P, Teutsch B, Frim L, Vancsa S, Kiss S, et al. Prophylactic transcatheter arterial embolization reduces rebleeding in non-variceal upper gastrointestinal bleeding: A meta-analysis. World J Gastroenterol. 2021;27(40):6985-6999.
doi pubmed - Yu Q, Liu C, Collura B, Navuluri R, Patel M, Yu Z, Ahmed O. Prophylactic transcatheter arterial embolization for high-risk ulcers following endoscopic hemostasis: a meta-analysis. World J Emerg Surg. 2021;16(1):29.
doi pubmed - Jensen DM, Kovacs TOG, Ohning GV, Ghassemi K, Machicado GA, Dulai GS, Sedarat A, et al. Doppler endoscopic probe monitoring of blood flow improves risk stratification and outcomes of patients with severe nonvariceal upper gastrointestinal hemorrhage. Gastroenterology. 2017;152(6):1310-1318.e1311.
doi pubmed - Zetner D, Rasmussen IR, Frykman CP, Jensen LR, Jensen RJ, Possfelt-Moller E, Taudorf M, et al. Risk factors for rebleeding and mortality following prophylactic transarterial embolization for patients with high-risk peptic ulcer bleeding: a single-center retrospective cohort study. Surg Endosc. 2024;38(4):2010-2018.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.