Eflornithine for the Chemoprevention of Luminal Gastrointestinal Neoplasms: A Systematic Review
DOI:
https://doi.org/10.14740/gr1801Keywords:
Chemoprophylaxis, Gastrointestinal luminal cancer, Eflornithine, DFMOAbstract
Background: Gastrointestinal (GI) tract malignancies represent a significant global health burden, being major contributors to cancer-related morbidity and mortality globally, with over 7.7 million cases reported. While aspirin is a well-studied chemopreventive agent for GI neoplasms, its use may be limited due to the underlying bleeding risk. Eflornithine (DFMO) is an inhibitor of the ornithine decarboxylase (ODC) which inhibits polyamine synthesis, and has shown promise as an alternative chemopreventive agent, particularly in animal studies and limited clinical trials.
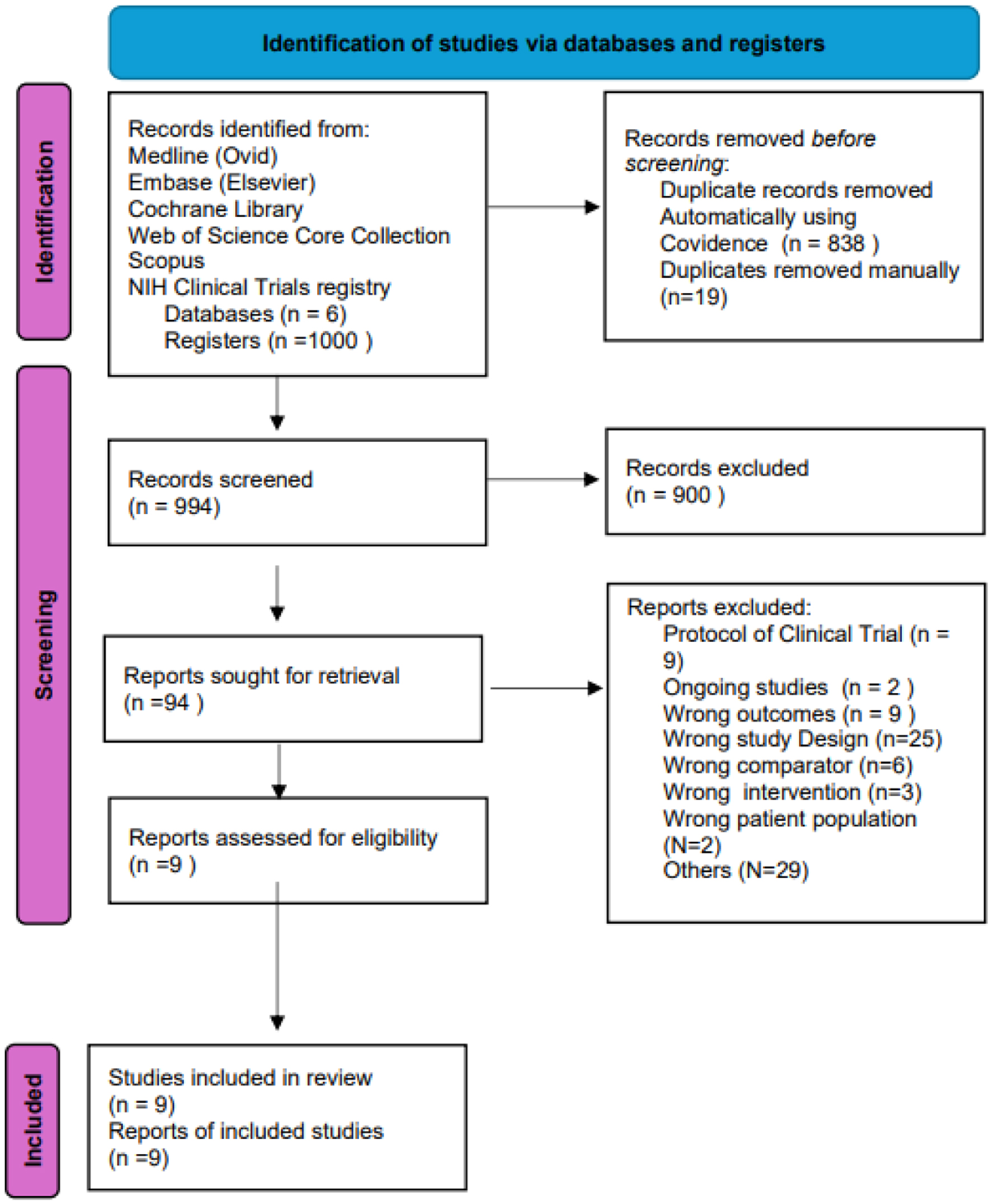
Methods: Following PRISMA guidelines, we conducted a systematic review of studies evaluating DFMO alone or in combination for chemoprevention in premalignant GI lesions including chronic gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia. The protocol was registered in Prospero (CRD42022309307). Randomized controlled trials (RCTs) and cohort studies in English or Spanish were included.
Results: Nine studies (six RCTs and three phase I-II trials) met inclusion criteria. Phase I-II trials involving Barrett’s esophagus and gastric cancer did not report significant benefits. Phase III-IV trials combining DFMO with nonsteroidal anti-inflammatory drugs (NSAIDs) were associated with reductions in adenoma recurrence, size, and polyamine levels in high-risk GI cancer populations. Side effects included ototoxicity, reversible upon discontinuation, and mild GI events, both occurring at higher doses.
Conclusion: While aspirin remains a frontline chemopreventive agent for GI neoplasms, this review shows that phase III-IV trials suggest promising outcomes in combination with NSAIDs, warranting further investigation. Notably, DFMO’s low cost and favorable toxicity profile may position it as a viable alternative, emphasizing the need for additional RCTs to delineate its efficacy and safety in GI cancer prevention. Further investigation into DFMO’s optimal dosage, duration, and side effect management is essential to establish it as a safe and effective chemopreventive agent.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.