Metabolic Regulatory Mechanism of Gastric Motility by Acetylcholine
DOI:
https://doi.org/10.14740/gr2063Keywords:
Gastric motility, Calcium-activated chloride channels, ATP-sensitive potassium channels, Acetylcholine-induced phasic contraction, Energy metabolismAbstract
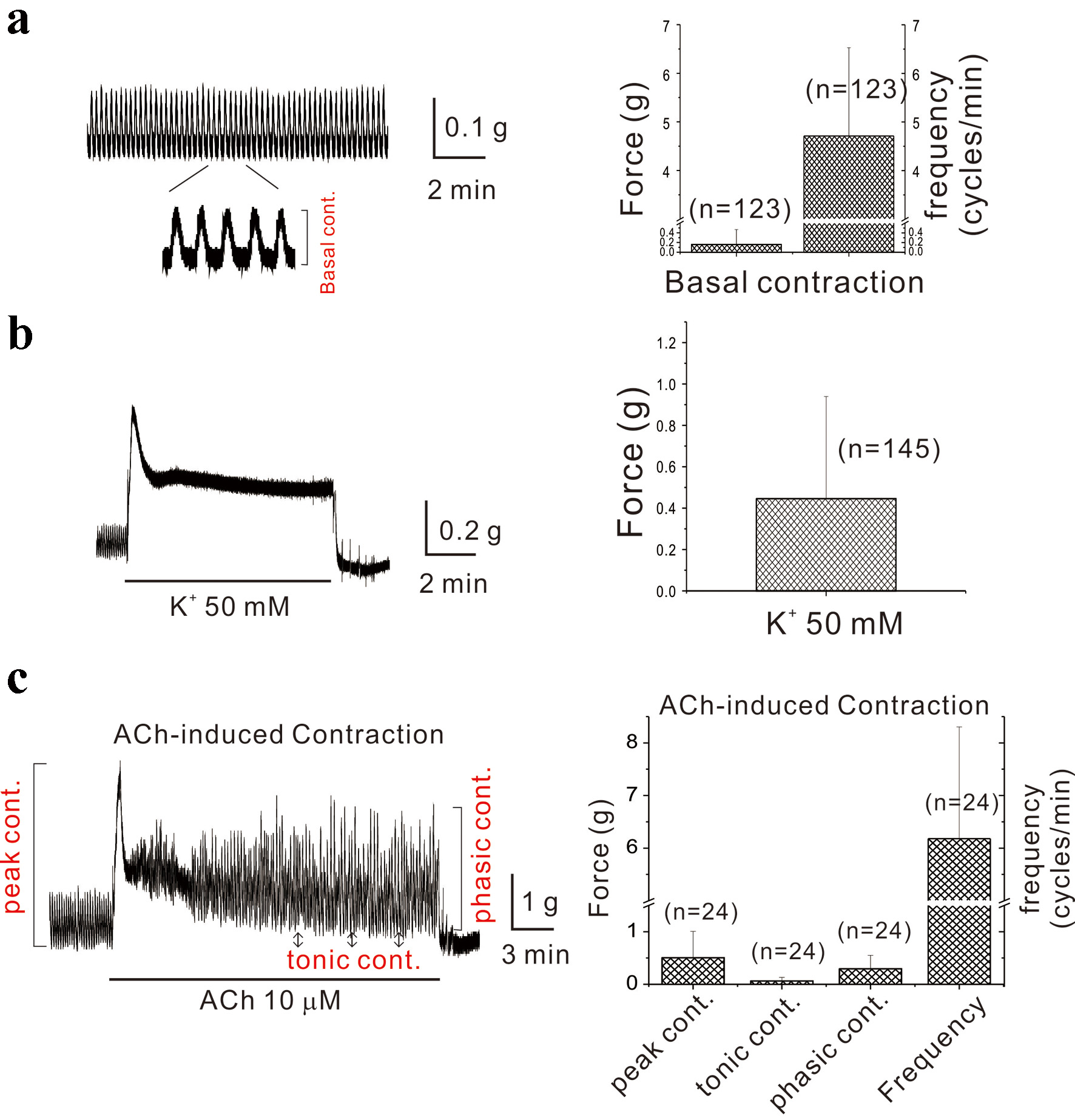
Background: Acetylcholine (ACh), a crucial neurotransmitter for gastric contractions, induces triphasic contraction in stomach. However, the precise mechanism of ACh-induced phasic contractions (AiPCs) remains unclear. Recent data suggest the chloride channel (Cl- channel) may be the principal channel for AiPC in the stomach. Previous studies demonstrated that the opening of the ATP-sensitive potassium (KATP) channel inhibits AiPC. However, it has not been studied whether inhibition of energy metabolism can regulate AiPC in gastric smooth muscles via this channel. This study investigated whether AiPC in gastric smooth muscle are mediated by chloride channels and modulated by KATP channels under conditions of energy metabolism inhibition.
Methods: Isolated gastric smooth muscle strips from mice and humans were used to record isometric contraction. The subunits of Cl- and KATP channels were evaluated by Western blot.
Results: Niflumic acid and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), known to block the Cl- channel, inhibited AiPC in gastric smooth muscles of mice. Sodium cyanide (NaCN) and dextro-mannitol, which inhibit energy metabolism, reduced AiPC in gastric smooth muscles of mice. NaCN also lowered AiPC in gastric smooth muscles of humans and vasomotion in human arterial smooth muscles. By Western blot, subunits of the KATP and Cl- channels were identified in gastric smooth muscles of mice and arterial smooth muscles of humans.
Conclusions: This is the first study to demonstrate that suppression of energy metabolism reduces AiPC through activation of KATP channels in both murine and human gastric smooth muscle, linking metabolic state to excitatory neurotransmission. Vasomotions in arterial smooth muscles of humans are also decreased by inhibition of energy metabolism.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









